Xanthine oxidase ñ allopurinol for gout
:
Xanthine oxidase is the last enzyme on the breakdown
pathway of purine bases in primates and it catalyses the conversion of
hypoxanthine to xanthine and of xanthine to uric acid. The latter is
normally excreted, although quantities of the other purines may also find
their way into the urine. In some diseases, notably gout, the production
of purines can be increased as a primary cause of the disease. Enzyme
deficiencies with a genetic origin may play a part. One such case is
deficiency of the salvage enzyme hypoxanthine phosphoribosyltransferase (HPRT)
which leads to an elevated level of hypoxanthine
phosphoribosylpyrophosphate. The latter stimulates de
novo purine biosynthesis at the initial rate-limiting step of the
formation of phosphoribosylamine.
The consequence of increased
purine synthesis is an increased throughput down the catabolic pathway to
uric acid. When levels of the latter rise above saturation, crystals of
monosodium urate form in the synnovial fluid. The characteristic symptoms
of gout derive from an inflammatory response to these crystals and thus
closely resemble the painful joint swellings in rheumatoid arthritis. This
may occur in one joint only or in several. In advanced gout, deposits (tophi)
of sodium urate form on or near joints or tendon sheaths, which are soft
initially but eventually harden.
For therapy the major need is to lower serum uric acid levels, although
anti-inflammatory drugs will relieve the symptoms on a short-term basis.
One of the most useful drugs in effecting a long-term cure is allopurinol.
Xanthine oxidase is the target of the drug, and so serum and urine
hypoxanthine and xanthine levels are raised while, more importantly, uric
acid levels are lowered. In addition, the drug is useful when given in
combination with anti-leukaemic drugs since serum urate levels can rise
sharply as the leukaemia cells die. This is an example of secondary gout,
secondary in that uric acid formation is increased as a consequence of
other changes. In this case, the danger is not only that acute episodes of
gout may occur, but also that sodium urate crystals may form in the distal
tubule of the kidney.
Clearly, if a drug is metabolised by
xanthine oxidase, its action is likely to be potentiated by allopurinol.
For example, 6-mercaptopurine (a drug used for the treatment of leukaemia)
is metabolized
by xanthine oxidase to 6-thiouric acid, an inactive
metabolite. The dose of mercatopurine required
when given in conjunction with allopurinol must therefore be reduced to
avoid widespread toxicity which would otherwise occur if higher
mercatopurine levels were sustained for longer periods of time.
Xanthine oxidase is a complex
enzyme containing, in effect, a transport system involving molybdenum,
flavin nucleotide and two iron-sulphur centres which convey electrons to
oxygen to yield superoxide ion (O-2). Allopurinol
inhibits the enzyme in a complex fashion, and may be regarded as one of
the earliest examples of a suicide substrate. If the inhibition is studied
without pre-incubation of enzyme and inhibitor, allopurinol behaves as
though it were as competitive inhibitor with a Ki of 7¥10-7
M. With pre-incubation in the presence of air, the inhibition increases
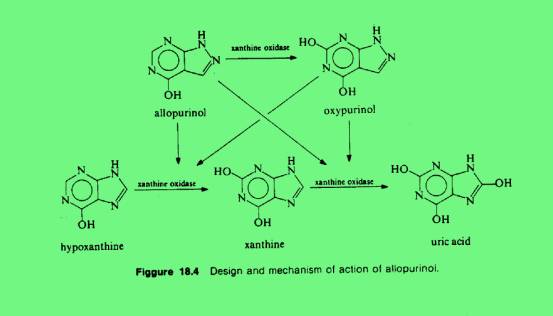
and it is no longer competitive with substrate. Allopurinol is also a
substrate for xanthine
oxidase and the product of the reaction, oxypurinol (alloxanthine), is
also an inhibitor. In the presence of xanthine as substrate and oxygen, or
anaerobically without substrate, the enzyme is inactivated by oxypurinol.
If the oxidation of xanthine, which require the enzyme to cycle between
reduced and oxidized forms, and for the enzyme to
be in an anaerobic environment, both result in enzyme inactivation
by oxypurinol, it is likely that the reduced form of the enzyme is
sensitive to oxypurinol. The dissociation constant of the oxypurinol-enzyme
complex is 5.4¥10-10
M. Inhibition can be reversed by prolonged dialysis or by allowing the
complex to be reoxidized in the presence of air, thus confirming that it
is the partly reduced form of the enzyme that is receptive to oxypurinol
inhibition.
The inactivation of reduced xanthine
oxidase by oxypurinol follows first-order kinetics by appearing to be
dependent on the concentration of reduced enzyme. This may be the result
of an internal rearrangement of the enzyme-inhibitor complex in a
time-dependent fashion. The similarity between the tight or stoichiomeric
binding of oxypurinol to xanthine oxidase, and of coformycin to adenosine
deaminase was noted by Cha et al (1975).
Allopurinol has been found to be effective in the treatment of kala-azar (leishmaniasis).
In this instance the drug is acting as a false substrate for the
parasiteís hypoxanthine phosphoribosyltransferase ñ much more
efficiently tan for the human erythrocyte enzyme. Subsequent enzymes
convert the ribonucleotide into an analogue of ATP which is then
incorporated into a faulty RNA3.
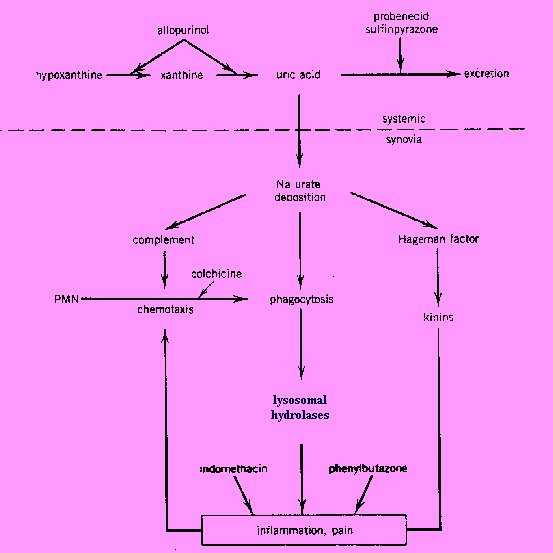
Mechanism of Action:
The
primary event in acute gouty arthritis is the local deposition of
crystalline monosodium urate hydrate. Ingestion of the crystals by
neutrophilic leukocytes leads to activation and release of lysosomal
enzymes. The negatively charged urate crystals also activate complement
and Hageman factor. The latter initiates the clotting mechanism and the
kinin cascade resulting in pain, increases of vascular permeability, and
accumulation of leukocytes.
Uricosurics, such as probenecid and sulfinpyrazone, promote the excretion
of uric acid by inhibiting the tubular reabsorption of filtered urate and
thereby lower the urate level in the blood. In consequence of this action,
tophi formation is decreased or prevented, exitsting urate deposits are
resolved, and after several months of treatment, the frequency of acute
attacks of gout is reduced.
Allopurinol, as well as its metabolic product oxypurinol, reduce the
biosynthesis of uric acid from xanthine. They act as inhibitors of
xanthine oxidase, the enzyme that converts hypoxanthine to xanthine and
xanthine to uric acid. Allopurinol binds 15 tims more tightly to xanthine
oxidase than its own natural substrate, xanthine. It inhibits also denovo
purine synthesis through a feedback mechanism in thoses patients who
possess the enzyme hypoxanthine-guanine phosphoribosyltransferase. By
decreasing both serun and urine concentrations of uric acid, allopurinol
and related compounds prevent or lower urate deposition and thereby hinder
then occurrence or progression of both urate nephropathy and gouty
arthritis. Patients with chronic gout may have prevented or decreased
tophi formation and chronic joint changes, resolved existing urate
crystals and deposits, and after several months of treatment, reduced the
frequency of acute attacks of gout1.
The most common side-effect of allopurinol is skin rash. Rashes are
generally maculopapular or pruritic, but more serious hypersensitivity
reactions may occur and include exfoliative rashes, the Stevens Johnson
syndrome, and toxic epidernal necrolysis. It is therefor recommended that
allopurinol be withdrawn immediately if a rash occurs. Further symtoms of
hypersensitivity include fever, chills, leucopenia or leucocytosis,
eosinophilia, arthralgia, and casculitis leading to renal and hepatic
damage. These hypersensitivity reactions may be severe, even fatal, and
patients with hepatic or renal impairment are at special risk.
Hepatotoxicity
and signs of altered liver function may be found in patients not
exhibiting hypersensitivity.
Many
other side-effects, usually of a less serious nature, have been noted and
include peripheral neuritis, alopecia, hypertension, taste disturbances,
nausea, vomiting, abdominal pain, diarrhoea, headache, drowsiness, and
vertigo.
In
addition to these adverse effects patients may experience an increase in
acute gouty attacks during the first few months of treatment.
A
Boston Collaborative Drug Surveillance Program of 29524 hospitalised
patients revealed that, with the exception of skin reactions, of 1835
patients treated with allopurinol 33 (1.8%) experienced adverse effects.
It appeared that although allopurinol is seldom associated with toxicity,
when it does occur it can be of a serious nature. Adverse effects were
dose-related and the most frequent were haematological (11 patients,
0.6%), diarrhoea (5 patients, 0.3%), and drug fever (5 patients, 0.3%).
Hepatotoxicity was reported in 3 patients (0.2%). Two patients developed
possible hypersensitivity reactions to allopurinol.
Another analysis involving 1748 outpatients indicated no instances of
acute blood disorders, skin diseases, or hypersensitivity that warranted
hospital treatment. Liver disease, although found, was considered to be
unassociated with allopurinol. There were only 2 patients in whom renal
disease could possibly have been caused by allopurinol.
Effects on the
blood. In addition to the
haematological abnormalities of leucopenia, thrombocytopenia, haemolytic
anaemia, and clotting abnormalities noted in the Boston Collaborative Drug
Surveillance Program, aplastic anaemia has also been reported, sometimes
in patients with impaired renal function.
Effects
on the endocrine system. A case of
male subfertility associated with allopurinol.
Effects on the eyes.
Although care reports have suggested an
association between allopurinol use and the development of cataracts, a
detailed ophthalmological survey which involved 51 patients who had taken
allopurinol failed to confirm this possible adverse effect.
Effects on the skin. Skin
reactions are generally accepted to be one of the most common side-effects
of allopurinol.
An Australian
report has calculated that 215 adverse effects noted over a 16-year period
188 (87.4%) were related to the skin or mucous membranes. An analysis by
the Boston Collaborative Drug Surveillance Program in the USA, of data on
15438 patients hospitalised between 1975 and 1982 detected 6 allergic skin
reactions attributed to allopurinol among 784 recipients of the drug.
Serious skin
reactions to allopurinol may occur. One report has described toxic
epidermal necrolysis which was clearly associated with allopurinol usage
in 5 patients (one fatality) and possibly associated in one further
patient. A fatality due to the Stevens-Johnson syndrome has also been
described and in this report it was aware at that time of 3 further cases
of the Stevens-Johnson syndrome probably due to allopurinol.
Allopurinol should not be used for the treatment of an acute attack of
gout; additionally, allopurinol therapy should nit be initiated for any
purpose during an acute attack. Treatment should be stopped if any skin
reactions or other signs of hypersensitivity develop. A cautions
re-introduction at a lower dose may be attempted when mild skin reactions
have cleared; allopurinol should not be re-introduced in those patients
who have experienced other forms of hypersensitivity reactions.
Allopurinol should be administered with care to patients with renal or
hepatic impairment, and doses may need to be reduced; further information
concerning dosage in the presence of renal impairment is provided under
Administration in Renal Failure in Uses and Administration, below. In all
patients receiving allopurinol it is advisable to maintain a urinary
output of not less than 2 litres a day and for the urine to be neutral or
slightly alkaline.
Allopurinol should be used with caution in nursing mothers as it
has been reported to be excreted in breast milk.
The metabolism of azathioprine and mercaptopurine is inhibited by
allopurinol and their doses should be reduced to one-quarter to one-third
of the usual dose when either of them is given with allopurinol. An
increase in hypersensitivity reactions, and possibly also other adverse
effects, has been reported in patients receiving allopurinol with thiazide
diuretics, particularly in patients with impaired renal function. There
have also been reports of allopurinol enhancing the activity of, and
possibly increasing the toxicity of, a number of other agents including
some antibacterials, some anticoagulants, some antineoplastics, captopril,
cyclosporin, theophylline, and vidarabine; further information concerning
these interactions is provided below. A number of drugs increase uric acid
concentrations and may require that the dose of allopurinol be adjusted.
Aspirin and the salicylates possess this activity and are avoided in
hyperuricaemia and gout.
ANTACIDS. Concurrent administration of
allopurinol with aluminium hydroxide
in patients on chronic harmodialysis has been reported to result in no
change to concentrations of uric acid in blood. However, if allopurinol
was given 3 hours before aluminium hydroxide the expected decrease in uric
acid concentration did occur.
ANTIBACTERIALS.
Although an increased incidence of skin rashes has been noted when
allopurinol has been used with ampicillin
or amoxycillin, data currently
available is insufficient to confirm whether this is due to allopurinol or
not.
ANTIGOUT
AGENTS. Concurrent administration of allopurinol and benzbromarone has been found to lower plasma concentrations of
oxypurinol (the major metabolite of allopurinol) by some 40%, although
plasma concentrations of allopurinol itself were not affected. The
interaction, which was thought to be due to accelerated clearance of
oxypurinol, probably due to reduced renal tubular reabsorption, was not
clinically significant, since the combination was more effective than
allopurinol alone in lowering serum concentrations of uric acid.
ANTIHYPERSENSITIVES.
An apparent interaction between allopurinol and captopril has been reported in patients with chronic renal failure.
In one patient fatal Stevens-Johnson syndrome developed and it was
suggested that the reaction was secondary to the introduction of
allopurinol potentiated by the presence of captopril.
In the second patient hypersensitivity, characterised by fever,
arthralgia, and myaglia, occurred and in this case the cause was believed
to be captopril, or one of its metabolites, potentiated by the addition of
combination of allopurinol and captopril should be prescribed with care,
especially in patients with chronic renal failure.
ANTINEOPLASICS
AND IMMUNOSUPPRESSANTS. Allopurinol inhibits the metabolism of azathioprine
and mercaptopurine. Other antineoplastics have also been involved in
interactions. Mild chronic allopurinol-induced hepatotoxicity has been
reported in one patient to have been exacerbated by tamoxifen.
Hypersensitivity vasculitis resulting in the death of one patient receving
allopurinol and pentostatin has
been described. Although it could not be ascertained whether this effect
was due to one of the drugs alone or to an interaction it was believed
that this combination should not be employed. For a report of an increased
incidence of bone-marrow toxicity in patients given allopurinol and cyclophosphamide.
Up to 90% of a dose of allopurinol is absorbed from the gastro-intestinal
tract after oral administration; its plasma half-life is
about 1 to 3 hours. Allopurinolís major metabolite is oxypurinol
(alloxanthine) which is also an inhibitor of xanthine oxidase with a
plasma half-life of about 15 or more hours in patients with normal renal
function, although this is prolonged by renal impairment. Both allopurinol
and oxypurinol are conjugated to form their respective ribonucleosides.
Allopurinol and oxypurinol are not bound to plasma proteins.
Excretion is mainly through the kidney, but it is slow since
oxypurinol undergoes tubular reabsorption. About 70% of a daily dose may
be excreted in the urine as oxypurinol and up to 10% as allopurinol;
prolonged administration may alter these proportions due ti allopurinol
inhibiting its own metabolism. The remainder of the dose is excreted in
the faeces. Allopurinol and oxypurinol have also been detected in breast
milk.
Allopurinol is used to treat hyperuricaemia associated with chronic gout,
urate nephropathy, recurrent cancer or cancer chemotherapy; it is not used
to treat acute attacks of gout and may exacerbate them if given during an
attack. Allopurinol is also used in the management of renal calculi due to
the deposition of calcium oxalate and of 2,8-dihydroxyadenine. It is an
ingredient of kidney preservation solutions. In addition allopurinol has
antoprotozoal activity and has been used in leishmaniasis and American
trypanosomiasis.
Allopurinolís
use in hyperuricarmia and gout derives from its inhibitory action on the
enzyme xanthine oxidase which results in a reduction of the oxidation of
hypoxanthine to xanthine and xanthine to uric acid. The urinary purine
load, normally almost entirely, uric acid, each with its independent
solubility. This results in the reduction of urate and uric acid
concentrations in plasma and urine, ideally to such an extent that
deposits of monosodium urate monohydrate or uric acid are dissolved or
prevented from forming. At low concentrations allopurinol acts as a
competitive inhibitor of xanthine oxidase and at higher concentrations as
a non-competitive inhibitor. However, most of its activity is due to the
metabolite oxypurinol which is a non-competitive inhibitor of xanthine
oxidase.
Allopurinol
is not used to treat an acute gout although it may prevent attacks. It
should not be given until an acute attack has subsided. In the first few
moths of treatment with allopurinol there may be an increase in acute
attacks due to the release of urate from tophi; it is therefore
recommended that treatment should be started with a low dose increased
gradually and that a nonsteroidal anti-inflammatory drug or colchicine
should also be given over the first few months.
A
suggested starting dose of allopurinol is 100 mg daily by mouth, gradually
increased by 100 mg for example at weekly intervals until the
concentration of urate in plasma is reduced to about 60 mg
per ml, this generally occurs within about 3 weeks. A daily dose range of
100 to 300 mg may be adequate for those with mild gout and up to 600 mg
for those with moderately severe gout; dose of up to 900 mg daily may be
necessary in some patients with very severe hyperuricaemia. In general
however, the usual maintenance dose will be in the range of 200 to 600 mg
daily and should be continued indefinitely. Up to 300 mg may be taken as a
single daily dose larger amounts should be taken in divided doses.
Allopurinol is best taken after food in order to reduce gastric irritation
and patients should maintain an adequate fluid intake; ideally patients
should have a neutral or slightly alkaline urine.
When
used for hyperuricaemia and the prevention of urate nephropathy associated
with cancer therapy 600 to 800 mg is given daily in divided doses
generally for 2 or 3 days and starting before the cancer treatment. A high
fluid intake is essential. Maintenance doses of allopurinol are then given
according to the response.
The
main use of allopurinol in children is for hyperuricaemia associated with
cancer or cancer chemotherapy or with enzyme disorders. The suggested dose
varies; in the UK a dose of 10 to 20 mg per kg body-weight daily is
recommended, while in the USA. The dose is 150 mg daily for children under
6 years of age and 300 mg daily for those aged 6 to 10 years, adjusted if
necessary after 48 hours.
Allopurinol
has been given as the sodium salt by intravenous infusion in sodium
chloride 0.9% or glucose 5% to patients (usually cancer patients) unable
to tale allopurinol by mouth. Doses have ranged from the equivalent of 300
to 700 mg of allopurinol everry 24 hours.
Allopurinol
through its inhibition of xanthine oxidase can block the development of
free radicals. This has led some workers to try allopurinol sodium in
solutions for the preservation of kidneys for transplantation such as UW
solution.
Excretion of allopurinol and its active metabolite oxypurinol is primarily
via the kidneys and therefore dosage may need to be reduced if renal
function is impaired.
In
the USA one manufacturer has suggested a daily dose of 200 mg for patients
with a creatinine clearance of 10 to 20 mL per minute and a maximum daily
dose of 100 mg for a clearance of under 10 mL per minute with
consideration begin given to a longer dosing interval if the clearance
falls below 8 mL per minute.
In
the UK the manufacturers have considered that schedules based on
creatinine clearances are unsatisfactory because of the imprecision of low
clearance values. Instead, it is suggested, that if facilities are
available for monitoring, the dose should be adjusted to maintain plasma-oxypurinol
concentrations below 100 mmol
per litre (15.2 mg
per ml).
Deficiency of the enzyme ornithine carbomoyltransferase can result in
severe central nervous system dysfucntion or even in death, and
identification of women at risk of being carriers of this genetic enzyme
deficiency has been described. The enzyme deficiency causes carbomoylphosphate to accumulate,
which stimulates the synthesis of orotidine. The test relies on the
administration of a single dose of allopurinol, which will, in carriers,
greatly increase the urinary excretion of orotidine.
Epilepsy.
Reduction in the frequency of seizures has
been described in some patients with severe or intractable epilepsy when
allopurinol was added to their existing anticonvulsant therapy. Although
the mode of action was not known it was noted that the patients were not
heperuricarmic and that allopurinol did not affect plasma concentrations
of existing anticonvulsants.
Gout
and hyperuricaemia. Allopurinol is
used for the prevention of chronic gout and hyperuricaemia, including that
associated with the tumour lysis syndrome, but has no role in the
treatment of acute attacks of gout.
Muscular
dystrophies. Muscular dystrophies
are a range of inherited myopathies in which there is progressive
degeneration of muscle fibres and associated muscle weakness. They are
classified according to the mode of inheritance. The most common type is
the fatal recessive X-linked Duchenne
muscular dystrophy (DMD) in which there is a deficiency in the
structural muscle protein dystrophin. There is no effective therapy that
affects the course of the various muscular dystrophies. Management is
mainly through the use of physiotherapy, supports, and surgery.
Controversy
has surrounded the use of allopurinol in Duchenne muscular dystrophy since
the initial favourable report by Thomson and Smith. Allopurinol was used
in an attempt to increase the ATP levels in muscle which are depleted in
this muscular dystrophy.
Organ
transplantation. Besides being used
as an ingredient of kidnet presercation solitions with the aim of
protecting the organ from free radicals, allopurinol has also been added
to the immunosuppressive treatment given to the patient after
transplantation, and is reported to reduce the frequency of acute
rejection.
Protozoal
infections. Beneficial results have
been reported in patinets with
visceral leishamaniasis when allopurinol was added to their therapy,
these studies involved patients who either had a poor or no response to
antimonial drugs or included untreated patients from areas where
unresponsive cases were frequent. Positive results in leishmaniasis have
also been described in patients with AIDS. Additionally, a good response
in American cutaneous leishmaniasis
has been reported.
The
selective antiparasitic action of allopurinol is believed to be due to its
incorporation into the protozoal, but not the mammalian, purine salvage
pathway. This leads to the formation of 4-aminopyrazolopyrimidine
ribonucleotide triphosphate, a highly toxic analogue of adenosine
triphosphate, that is incorporated into ribonucleic acid. This action of
allopurinol is shared by allopurinol riboside, one ofthe minor
metabolities in man but not by oxypurinol, the major human metabolite.
Thus, some studies are now being conducted with allopurinol riboside,
rather than allopurinol, in an attempt to enhance activity by avoiding
host-mediated inactivation.


![]()