| |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
Synthesis for the last two nitro cubanes-
heptanitrocubane and octanitrocubane |
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
| |
HpNC |
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
| |
Interfacial nitration is not sufficient to further
nitration for heptanitrocubane. Al though it is very good in deed, we need
to find something which can successfully convert heptanitrocubane (HpNC). |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
| |
|
 |
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
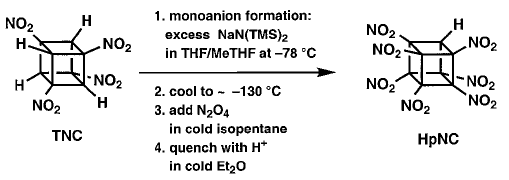
In this procedure TNC was treated with at least
4 equivalents of the base NaN(TMS)2 (where TMS = trimethylsilyl)
at ±78 �C in 1:1 THF/MeTHF. After the mono sodium salt had formed,
the solution was cooled to between ±125 and ±130°�C giving
a clear, but very viscous fluid. This was stirred vigorously as excess N2O4
in cold isopentane was added. After one minute, the base was quenched, and
the whole mixture was added to water. This resulted reproducibly in almost
complete conversion of TNC (1 g scale) to HpNC (95% by NMR), isolated crystalline
in 74% yield! |
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
ONC |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
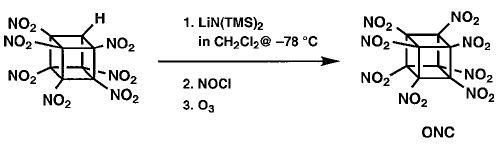
However, even in the presence of excess nitrating
agent (N2O4 or many others) no indication
of any formation of ONC was ever seen. It is suspected that anion nitration
with N2O4 proceeds by oxidation of the carbanion to
the corresponding radical.Perhaps the anion of HpNC is too stabilized for
this to occur. (HpNC is significantly ionized in neutral methanol.) This
concept led to the use of the more powerful oxidant nitrosyl chloride. Addition
of excess NOCl to a solution of the lithium salt of HpNC in dichloromethane
at 78° �C followed by ozonation at 78° �C gave the long-sought ONC
in 45±55% isolated yield on millimole scale. The intermediate product
prior to oxidation is thought to be nitrosoheptanitrocubane. |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
 |
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Finally, the magic molecule, the so called
the impossible molecule, octanitrocubane was synthesised. But, how good
are they and how useful are they? Let us discuss about it in the following
section. |
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|

