Structure determination from NMR data
First, the preferred conformations of the diastereomers 2a and 2b were calculated using MM+ force field, and it appeared that the C-H bond of phenylethylamine moiety is almost coplanar with the carbonyl group, so that the phenyl group lies above the plane of the heterocyclic ring.
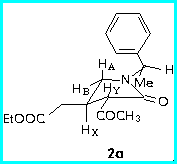
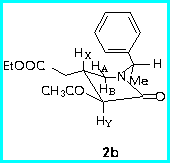
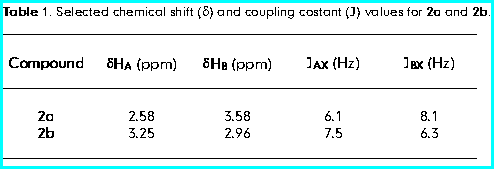
The chemical shifts and the coupling constants were diagnostic of the configurational assignment of C-4.
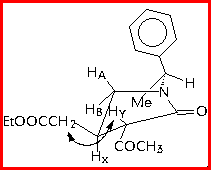
The trans-relationship between the substituents at C-3 and C-4 was established by JXY value and a large NOE effect between HY and the methylenic protons of CH2COOEt at C-4.
Return to the poster main page





