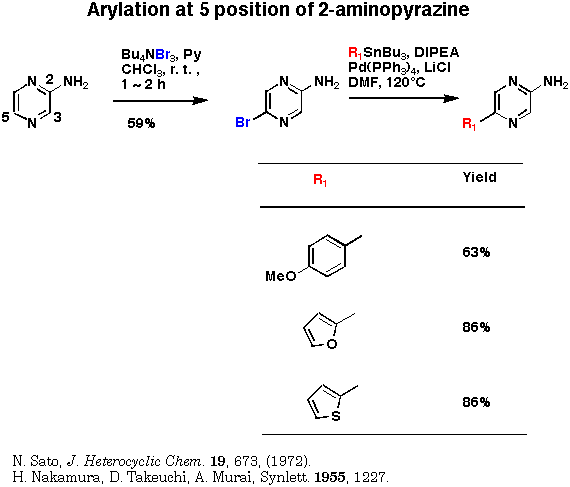

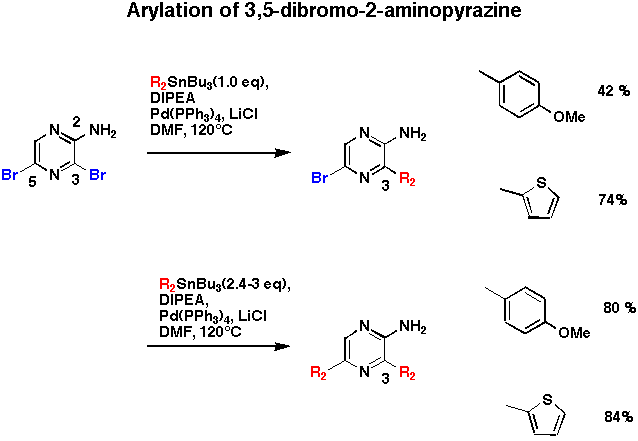
3,5-Dibromo-2-aminopyrazine prepared by Sato's method gave 3-aryl or 3,5-diaryl derivatives depending on the amount of arylstananes. The higher reactivity of the 3 position might be explained by the chelation effect of an amino group at 2 position.
 Home
Home