
As pointed out in the introduction, the objective for
producing U-shaped cavity systems has been associated with projects
involving host-guest complexation and nanotechnology. Accordingly,
we have targeted the production of bis-porphyrins and bis-(crown
ethers) as receptor sites for our guest molecules. In this presentation,
I will present our work on the production of bis(crown
ethers) emphasising the methodology used in their construction
rather than their application.
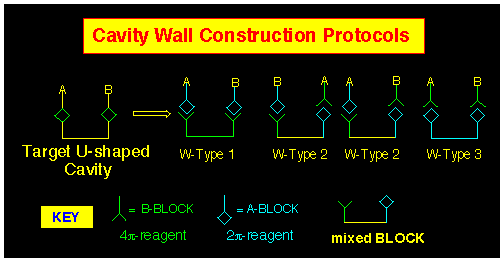
In order to emphasise the methodology, we have classified approaches
to the cavity systems in terms of those that involve attachment
of walls to existing short-walled systems using ACE or related
BLOCK techniques to achieve this goal (Figure 9).
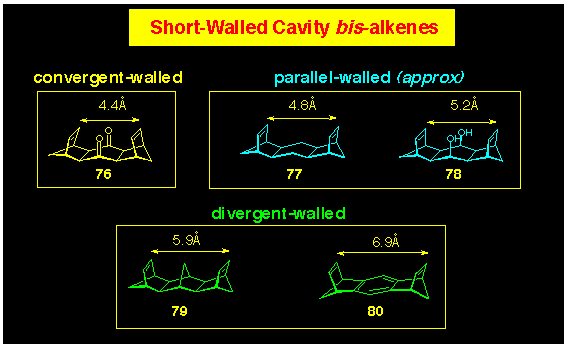
A further driving force in selecting this approach, has been
the range of short-walled bis-alkenes available to this
program (Figure 10), all of which have all been described in the
literature. Molecular modelling confirms the regular graduation
in the relative orientation of the p-bonds.
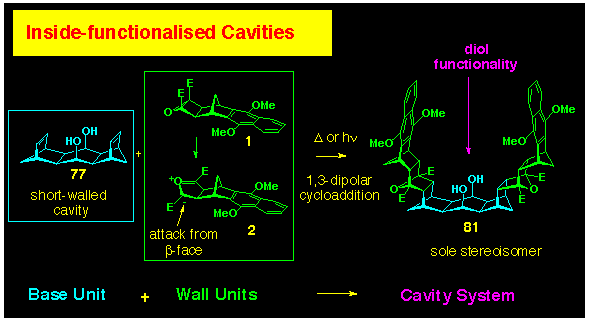
ACE reaction of the naphthalene functionalised cyclobutene epoxide
BLOCK 1 with the dihydroxy bis-alkene
77 gave the cavity systems 81 without incident (Scheme
24). This reaction could be achieved under either thermal conditions
(DGM, 140 oC, sealed tube) or photochemically
(acetone, Rayonet reactor, 300 nm). The structure of the cavity
system 81 was confirmed by NMR spectroscopy, where the
simplicity of the spectrum was in harmony with the C2v
symmetry of the product. Separate nOe experiments confirmed that
the expected exo,exo-stereoselectivity had occurred in
the coupling step leading to this cavity system.
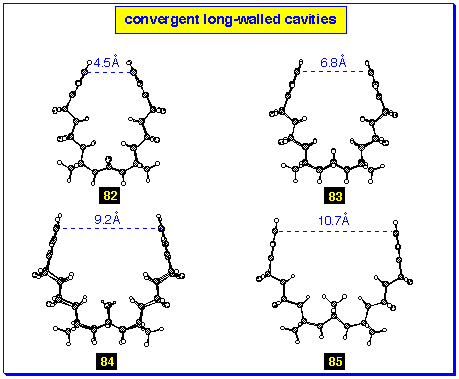
Molecular modelling studies show that the geometry of the starting
short-walled bis-alkenes 76-80 were not good
guides to the geometry of the final cavity systems 82-86.
For example, modelling of the starting dihydroxy bis-alkene
78 (Figure 10) indicates that the walls are roughly parallel,
whereas the modelling of the final product 84 (Figure 11)
indicates that the walls are decidedly convergent. Indeed, to
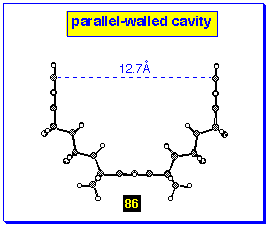
obtain a cavity product in this series with parallel walls, eg
86 (Figure 12), molecular modelling shows that it is necessary
to start from the short-walled bis-alkene 80 with
divergent walls.
These conclusions are based on molecular modelling results and we are currently seeking X-Ray crystallographic evidence to support this.
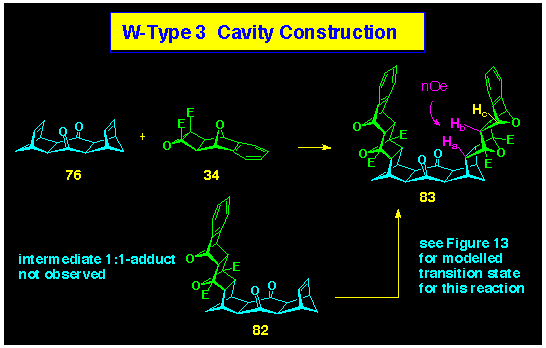
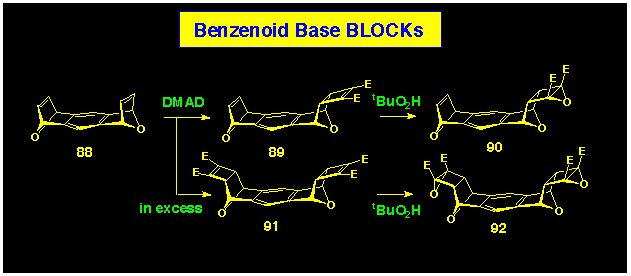
Heating the bis-alkene 76 with the cyclobutene
epoxide 34 produced the 1:2 cycloadduct 83 in good
yield (Scheme 25). The convergent U-shaped geometry present in
7 resulted from the stereospecific exo,exo-coupling
characteristic of the ACEcoupling procedure and was confirmed
by NMR spectroscopy. The high symmetry of product 83 was typified,
inter alia, by the single resonance of the four benzylic
bridgehead protons Hc at d
5.15 and the single resonance for the ester methyl groups (d
3.95). The stereochemistry was fully defined by the presence of a nOe between protons Hb and Ha which establishes their
proximity as required for the proposed exo, exo-geometry.
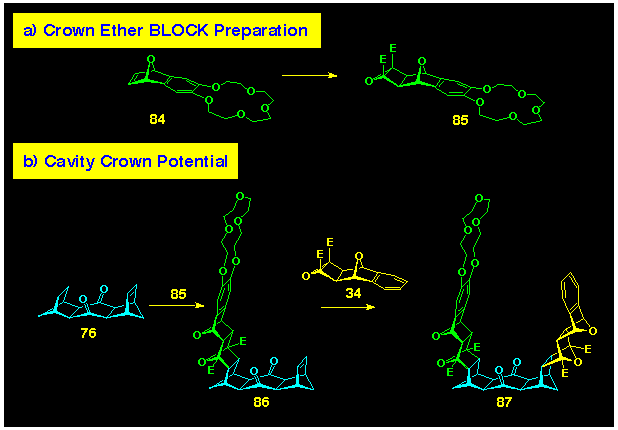
This effect of wall convergency manifested itself when the same
bis-alkene 76 was reacted with the crown ether containing
cyclobutene epoxide 87. Here, reaction stopped after the
addition of only one equivalent of the BLOCK
to produce the monocrown 90 (Scheme 26). We believe that
this indicates strong steric interaction between the terminal
crown ether units occurs in the transition state for bis-crown
formation, and this is supported by modelling of the transition
state for ACE cycloaddition onto the p-bond
of bis-alkene 76, which is shown in Figure 13a, Section 5.
This premise finds experimental support from the reaction of 1:1-adduct
90, which still contains a single p-bond,
with the smaller cyclobutene epoxide 34 which yielded
the unsymmetrically substituted cavity system 91. Molecular modelling of the transition state for a similar addition to the more open bis-alkene 79 are shown in Figure 14a (Section 5) and indicated that crown ether cavity formation should proceed. The experimental verification is yet to be conducted.
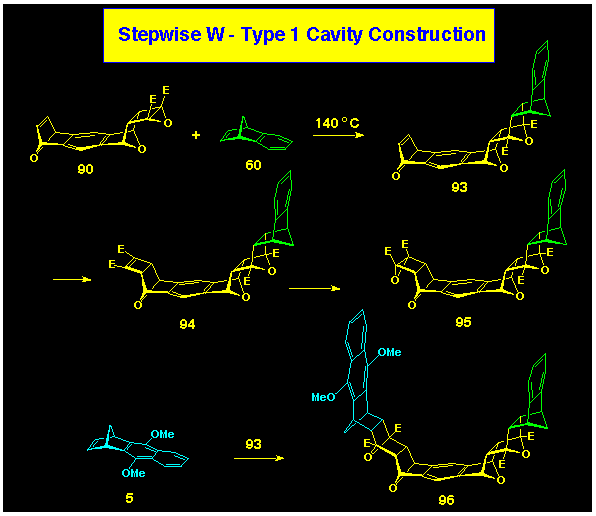
We have also investigated the more open bis-alkene 80
and its B-BLOCK derivatives 93 and 95 as ACE
reagents (Scheme 27) for cavity formation.
As a way of further developing our ability to make unsymmetrically-walled
cavity structures, we have used the mono-cyclobutene epoxide 93
as the prototype AB-BLOCK which contains both A and B types of
end-group.
This type of AB-BLOCK can be utilised in two ways:
a) it can be reacted in sequence with an alkene A-BLOCK followed
by a cyclobutene epoxide B-BLOCK (or vice versa) to produce a
cavity structure which contains the same or different wall units.
b) following reaction with an alkene A-BLOCK to produce the single-walled
product, the remaining alkene present can be elaborated to a new
cyclobutene epoxide and reacted with a second alkene A-BLOCK.
Each of these protocols has been explored and shown to be viable.
The viability of protocol a) to form cavity systems with different
wall units is confirmed by reaction of 96 with cyclobutene
epoxide 1 to furnish cavity 99, identical with that
produced via protocol b).
Protocol b) is illustrated by the reaction of the epoxide linking
point in 93 with benzonorbornadiene to produce the L-shaped
product 96. Elaboration of the norbornene p-bond
in 96 follows standard lines to produce cyclobutene 97
which yields the new epoxide 98 upon epoxidation. Coupling
of 98 with naphthonorbornadiene 5 yielded the cavity
system 99.
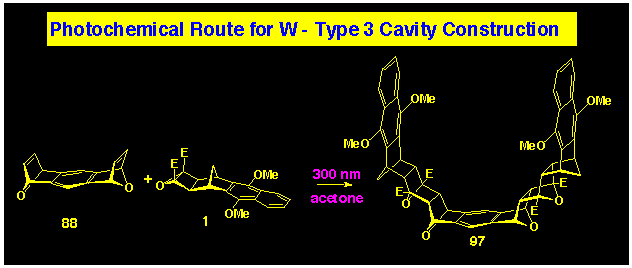
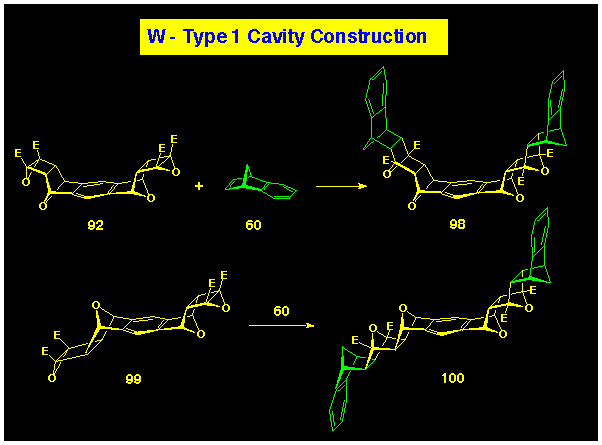
Direct ACE coupling of B-BLOCK 1 with bis-alkene
80 can be used to prepare the naphthalene-walled cavity
system 100.
The symmetrical cavity system 86 has been produced from
reaction of benzonorbornadiene 60 with the bis-(cyclobutene
epoxide) 95 to form the parallel-walled product 86
using the reverse assembly protocol where the wall units are supplied
as the A-BLOCK component (Scheme 30). The same protocol can be
applied to the anti-isomer of the bis-epoxide 101
to produce the stretched variant 102 where the walls
are still parallel, but anti-related about the benzenoid
base.
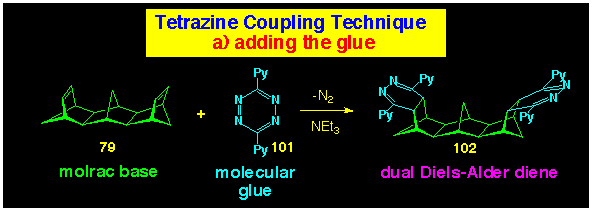
The s-tetrazine coupling route to cavity bis(crown
ethers) has also been explored, since molecular modelling of the
transition state (see Figure 13, Section 5) for the second, Diels-Alder cycloadditions in
the C17 bis-alkene 79 has the crown ether
components well separated, thereby avoiding the steric crowding
observed in the inward-facing bis-alkene 76 (Scheme
26). Satisfyingly, the reaction of the bis(dihydropyridazine)
102 formed by reaction of 79 with 3,6-di(2-pyridyl)
s-tetrazine 103 in the presence of triethylamine,
(see Scheme 31 for preparation) with the crown benzonorbornadiene
83 yielded the cavity bis(crown ether) 105 (Scheme
32).
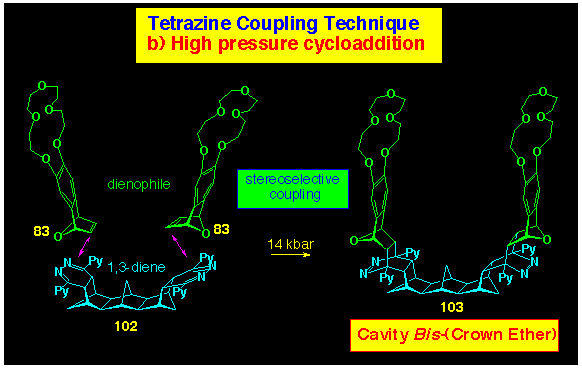
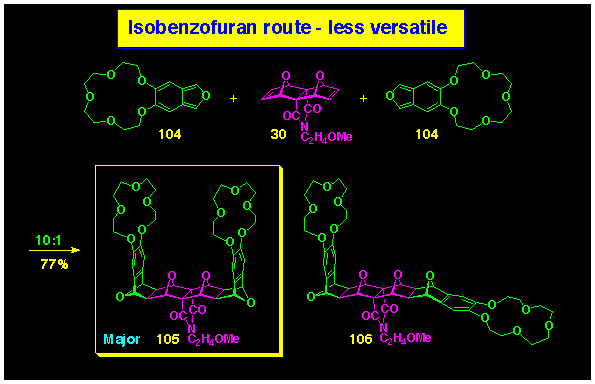
This simple entry to U-shaped bis-crowns complements our
earlier report to such compounds utilising a Diels-Alder cycloaddition
of crown isobenzofuran 104 directly onto the bis-alkene
30 which produced the cavity crown 107 admixed with
its L-shaped isomer 108, see Scheme 33. This methodology
is restricted to 7-oxanorbornene compounds in order to achieve
the required exo, endo-stereoselectivity and is consequently
less versatile than the BLOCK coupling procedure.
In addition, it is not suitable for the clean production of unsymmetrical
systems.