4. Experimental

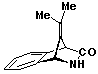
1,2-Dihydro-9-(1-methylethylidene)-1,4-methanoisoquinolin-3(4H)-one 25
3,6-Di(2-pyridyl)stetrazine (10.0g; 42.0 mmol) was added slowly to a stirred solution of the hydrocarbon 33 (7.0g; 38.4 mmol) and tosyl cyanide (7.0g; 38.6 mmol) in chloroform (150 ml), the resulting solution stirred for 1 hour and the solvent removed. The residue was dissolved in acetic acid (100 ml), stirred for 3 hours and poured onto a mixture of ice and water (100 g). The resulting mixture was basified with solid sodium hydroxide and the precipitate filtered off and washed with hydrochloric acid (2 x 50 ml; 0.5M) and water (3 x 50 ml). Recrystallization from ethanol (2x) afforded the product as fine colourless crystals (4.6g; 60%), m.p. 243-244 oC (dec).
uv, visible spectrum: lmax 238, 265, 271, 278 nm; e 3150, 1010, 1490, 1630.
infra red spectrum: umax 3380 (s), 1665 (s), 1225 (m), 1150 (m), 1090 (w), 980 (m), 935 (w), 900 (w), 875 (w), 840 (w), 810 (w), 775 (w), 730 (m), 710 (m), 670 (m) cm-1.
P.m.r. spectrum (CDCl3): d 1.63 (3H, s, -CH3), 1.70 (3H, s, -CH3), 4.18 (1H, d Ja,c = 2 Hz, Hc), 5.12 (1H, d Ja,c = 2 Hz, Ha), 6.20 (1H, exchanged with -OD/D2O, Hb), 7.08-7.56 (4H, m, aromatic protons).
Mass spectrum: m/e 199 (M+, 0.3%), 157(15), 156(100), 155(18), 154(5), 153(6), 142(10), 141(75), 139(9), 129(7), 128(15), 127(6), 116(7), 115(21), 77(6), 63(6), 51(5), 28(12), all other peaks less than 5%.
Analysis Found: C, 78.0; H, 6.5; N, 6.9. C13H13NO
requires C, 78.4; H, 6.6; N, 7.0%).
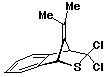
3,3-Dichloro-3,4-dihydro-9-(1-methylethylidene)-1,4-methano-1H-2-benzothiopyran 35
3,6-Di(2-pyridyl)stetrazine (0.45g; 1.9 mmol) was added slowly to a stirred solution of the hydrocarbon 35 (0.30g; 1.6 mmol) and thiophosgene (0.125 ml; 1.6 mmol) in chloroform (10 ml) and the resulting solution stirred for 15 minutes. The solvent was removed and the residue extracted with boiling light petroleum (3 x 10 ml). The extract was filtered, rapidly washed with hydrochloric acid (3 x 20 ml; 0.5M) and water (2 x 10 ml), dried, and its volume reduced to ca 5 ml. The product crystallized from this solution as redbrown needles. Recrystallization from light petroleum afforded the product as colourless needles (0.39g; 87%).
P.m.r. spectrum (CDCl3): d 1.74 (3H, s, methyl protons), 1.78 (3H, s, methyl protons), 4.72 (1H, d Ja,b = 1.5 Hz, Hb), 5.12 (1H, d Ja,b = 1.5 Hz, Ha), 7.12-7.60 (4H, m, aromatic protons).
Mass spectrum: (M+, m/e 270.0034. C13H1232S35Cl2 requires 270.0036;
m/e 272 (8%), 270(M+, 11), 237(18),
236(41), 235(39), 234(93), 222(6), 221(44), 220(20), 219(100),
201(25), 200(30), 199(99), 198(11), 197 (10), 186 (7), 185(16),
184 (68), 183(9), 171(7), 167(5), 166(24), 165(27), 156(26), 155(10)
153(8), 152(17), 151(6), 141(17), 140(6), 139(27),128(6), 127(5),
117(7), 115(7), 113(5), 99(7), 92(15), 91(10), 89(5), 79(8), 76(5),
75(6), 63(10), 62(5), 51(6), 45(5), 41(6), 39(10), 36(8), all
other peaks less than 5%.
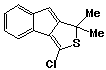
1,1-Dimethyl-3-chloro-1H-indeno[1,2-c]thiophene 42
The crude thiophosgene adduct 35, prepared as above, was chromatographed on a column of alumina (4 cm dia., 100g) using a 1:1 mixture of chloroform and light petroleum as eluent. The rearranged compound 42 was recovered as a yellow coloured oil and purified by rapid preparative thinlayer chromatography (silica, light petroleum) to yield solid material. Recrystallization from chloroform afforded the product as fine yellow coloured crystals (0.16g; 44%); m.p. 39-40 oC.
uv, visible spectrum: lmax 235 (inf), 239, 248, 275(inf), 283, 286(inf), 292(inf), 294(inf), 348, 359(inf) nm; e 11770, 12670, 10450, 7410, 9050, 7900, 8890, 10200, 12840, 12010.
infra red spectrum: umax 3060(w), 1620 (m), 1595 (s), 1575 (w), 1560 (m), 1445 (s), 1330 (w), 1225 (w), 1190 (m), 1155 (w) 1125 (w), 1019 (w), 985 (s), 955 (m), 860 (s), 820 (m), 760 (m), 745 (s), 695 (s) cm-1.
P.m.r. spectrum (CDCl3): d 1.74 (6H, s, methyl protons), 6.25 (1H, s, Ha), 7.0-7.7 (4H, m).
Mass spectrum: (M+, m/e 234.0272. C13H1132S35Cl requires 234.0270);
m/e 237 (7%), 236(39), 235(18), 234(M+,
100), 222(6), 221(40), 220(16), 219(100), 201(10), 216(16), 199(86),
198(9), 197(8), 186(6), 185(10), 184(55), 183(7), 166(19), 165(23),
153(5), 152(12), 139(20), 132(11), 131(7), 117(6), 115(9), 63(6),
28(12), all other peaks less than 5%.

3,3-Dichloro-3,4-dihydro-9-(diphenylmethylidene)-1,4-methano-1H-2-benzothiopyran 46
The title compound was prepared by the above method from the hydrocarbon 44 (0.50 g; 1.6 mol) and thiophosgene. Recrystallization from light petroleum afforded the product46 as colourless crystals (0.59g; 92%).
P.m.r. spectrum (CDCl3): d 4.81 (1H, d Ja,b = 1.5 Hz, Hb), 5.21 (1H, d Ja,b = 1.5 Hz, Ha), 7.05-7.70 (14H, m, aromatic protons).
Mass spectrum: (M+, m/e 394.0350. C23H1632S35Cl2 requires 394.0350);
m/e 396 (11%), 394(M+, 12), 362(5),
361(21), 360(25), 359(53), 358(29), 327(7), 326(5), 325(10), 324(22),
323(50), 321(8), 293(8), 291(22), 290(20), 289(30), 287(5), 281(17),
280(52), 279(25), 278(10), 277(11), 276(16), 252(6), 245(5), 203(19),
202(21), 200(5), 165(7), 161(7), 145(10), 86(5), 79(5), 78(15),
71(8), 70(7), 69(5), 57(36), 56(30), 55(12), 51(6), 43(68), 42(44),
41(52), 39(20), 38(90), 37(14), 36(100), 35(43), 29(30), 28(17),
27(27), all other peaks less than 5%.
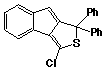
1,1-Diphenyl-3-chloro-1H-indeno[1,2-c]thiophene 47
The above procedure, for the preparation of 42, was followed, using the crude thiophosgene adduct 46. Recrystallization from chloroform afforded the product as yellow coloured crystals (0.37g; 65%), m.p. 161-162 oC.
uv, visible spectrum: lmax (cyclohexane) 244, 279 (inf), 281, 295 (inf), 298, 338 (inf), 364 nm; e 20650, 7150, 9270, 7810, 8460, 5690, 11220.
infra red spectrum:umax 3060 (w), 1615 (m), 1590 (s), 1570 (m), 1530 (m), 1495 (m), 1445 (s), 1430 (m), 1325 (w), 1205(w), 1165 (w), 1145 (w), 1080 (w), 1020 (m), 1005 (m), 960 (s), 870 (w), 840 (m), 825 (m), 805 (m), 775 (m), 755 (m), 745 (s), 720 (m), 700 (s) cm-1. P.m.r. spectrum (CDCl3): d 6.44 (1H, s, Hc), 7.00-7.70 (13H, m, Hb & phenyl protons), 7.80-8.00 (1H, m, Ha).
Mass spectrum: (M+, m/e 358.0584. C23H1532S35Cl requires 358.0583);
m/e 360 (4%), 358 (M+, 10), 324(5),
323(15), 307(27), 306(100), 305(50), 304(10), 303(13), 302(11),
293(5), 292(21), 291(17), 290(25), 286(15), 281(5), 279(8), 278(8),
277(9), 276(14), 230(15), 229(77), 228(50), 228(9), 226(15), 215(16),
213(8), 212(14), 191(17), 189(10), 179(9), 178(35), 176(8), 165(10),
153(6), 152(10), 151(10), 150(6), 144(10), 139(9), 138(12), 128(22),
126(7), 115(9), 113(5), 77(5), 51(7), all other peaks less than
5%.
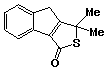
1,1-Dimethyl-3,8-dihydro-1H-indeno[1,2-c]thiophen-3-one 48
3,6-Di(2-pyridyl)stetrazine (0.45g; 1.9 mmol) was added slowly to a stirred solution of the hydrocarbon 33 (0.30g; 1.6 mmol) and thiophosgene (0.125 ml; 1.6 mmol) in a 1:1 mixture of ethanol and chloroform (10 ml) and the resulting mixture stirred for 15 minutes. The solvent was removed and the residue partitioned between ether and water. The ethereal phase was washed with hydrochloric acid (3 x 20 ml; 0.5M) and water (2 x 10 ml), dried, and the solvent removed to yield the crude product. Purification by rapid preparative thinlayer chromatography (silica, chloroform/light petroleum 2:1) yielded a pale brown coloured oil which crystallized from ethanol. Recrystallization from ethanol afforded the product as clear, pale brown crystals (0.18g; 52%), m.p. 66-67 oC.
uv, visible spectrum: lmax 238, 243 (inf), 263 (inf), 300 (inf), nm; e 12650, 12080, 5120, 1490.
infra red spectrum: umax 1695 (s), 1315 (w), 1305 (m), 1270 (m), 1240 (m), 1200 (w), 1185 (w), 1145 (m), 1120 (m), 1080 (w), 1065 (m), 1015 (w), 870 (w), 855 (w), 835 (s), 780 (m), 760 (m), 720 (s), 690 (m) cm-1.
P.m.r. spectrum (CDCl3): d 1.82 (6H, s, methyl protons), 3.54 (2H, s, benzylic protons), 7.20-7.60 (3H, m, Hb), 7.70-7.90 (1H, m, Ha),
13C-[1H] nmr spectrum (CDCl3): d 29.12 (C9 & C10), 33.70 (C8), 55.90 (C1), 121.04 (C5), 124.91 (C6), 126.25 (C4), 127.12 (C7), 136.05 (C3b), 143.16 (C3a), 145.89 (C7a), 181.25 (C8a), 191.33 (C3).
Mass spectrum: m/e 217 (10%), 216 (M+, 61), 215(5), 201(15), 189(6), 188(29), 187(20), 183(9), 173(14), 171(6), 158(8), 157(26), 156(14), 155(33), 153(8), 152(7), 142(22), 141(18), 139(14), 130(6), 129(38), 128(19) 127(10), 116(7), 115(38), 114(20), 113(8), 102(7), 89(9), 88(7), 87(9), 86(8), 85(5), 79(9), 77(12), 76(10), 75(10), 74(10), 70(5), 69(5), 65(8), 64(10), 653(27), 62(13), 61(8), 59(100), 51(21), 50(11), 45(14), 41(12), 39(30), all other peaks less than 5%.
Analysis: Found: C, 71.9; H, 5.8, S, 14.9. C13H12OS
requires C, 72.2; H, 5.6; S, 14.8%.
Reaction and rearrangement of 3,3-dichloro-3,4-dihydro-9-(1-methylethylidene)-1,4-methano-1H-2-benzothiopyran 35 in ethanol
The thiophosgene adduct 35, prepared as above, was dissolved in a 2:1 mixture of ethanol and chloroform, stirred for 3 hours, and the solvent removed. Purification by preparative thinlayer chromatography (silica, chloroform/light petroleum 1:1) afforded two products.
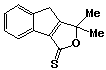
1,1-Dimethyl-3,8-dihydro-1H-indeno[1,2-c]furan-3-thione 49, less polar band, was recrystallized (2x) from ethanol to yield clear, yellow crystals (0.04g; 12%), m.p. 8384 oC.
uv, visible spectrum: lmax 247, 257, 276 (inf), 287(inf), 313 nm; e 8600, 7320, 3840, 4260, 5260.
infra red spectrum:umax (1585 (m), 1565 (m), 1420 (m), 1315 (m), 1245 (s), 1185 (m), 1175 (m), 1155 (m), 1135 (m), 1095 (w), 1065 (s), 985 (m), 970 (m), 865 (w), 825 (w), 760 (s), 730 (s), 665 (w) cm-1.
P.m.r. spectrum (CDCl3): d 1.74 (6H, s, methyl protons), 3.58 (2H, s, benzylic protons), 7.20-7.64 (3H, m, Hb), 8.00-8.15 (1H, m, Ha).
13C-[1H] nmr spectrum (CDCl3): d 25.23 (C9 & C10), 32.99 (C8), 94.23 (C1), 121.07 (C5), 124.86 (C6), 126.63 (C4), 127.25 (C7), 135.21 (C3b), 146.16 (C3a & C7a), 176.29 (C8a), 204.24 (C3).
Mass spectrum: m/e 218 (6%), 217(17), 216(M+, 100), 215 (5), 201(12), 189(11), 188(62), 187(45), 183(9), 174(5), 173(28), 172 (5), 171(8), 158(9), 157(20), 156(15), 155(60), 154(7), 153 (12), 152(6), 142(13), 141(27), 139(11), 129(30), 128(15), 127(9), 116(6), 115(34), 114(12), 113(6), 89(5), 77(6), 76(5), 63(8), 59(17), 51(6), 45(6), 43(16), 39(7), 32(5), 28(18), all other peaks less than 5%.
Analysis: Found: C, 72.1; H, 5.6; S, 14.8. C13H12SO
requires C, 72.2; H, 5.6; S, 14.8%.
1,1-Dimethyl-3,8-dihydro-1H-indeno[1,2-c]thiophen-3-one 48, more polar band, was recrystallized (2x) from ethanol to yield clear, pale brown crystals (0.17; 49%), m.p. 6667 oC.
When this reaction is carried out in a 2:1:1 mixture of ethanol,
chloroform and hydrochloric acid (2M) (15 ml), the reaction goes
to completion within 1 hour and the ratio of thione : ketone increases
to 1:1.
Generation of 8,8-dimethyl-isobenzofulvene in the presence of thiophosgene and ethanol
3,6-Di(2-pyridyl)stetrazine (0.45g; 1.9 mmol)
was added slowly to a stirred solution of the hydrocarbon 33
(0.30g; 1.6 mmol) and thiophosgene (0.125 ml; 1.6 mmol) in a 1:1
mixture of ethanol and chloroform (10 ml) and the resulting mixture
stirred for 15 minutes. The solvent was removed and the residue
chromatographed on a column of basic alumina (2 cm dia., 50g,
activity III) using light petroleum as eluent. Two compounds
were recovered.
1,1-Dimethyl-3-chloro-1H-indeno[1,2-c]thiophene
42 was obtained in 5% yield (20 mg).
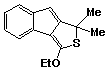
1,1-Dimethyl-3-ethoxy-1H-indeno[1,2-c]thiophene 50 was obtained as a yellow oil (0.14g; 38%).
P.m.r. spectrum (CDCl3): d
1.50 (3H, t, J = 8 Hz, -CH2CH3),
1.78 (6H, s, methyl protons), 4.34 (2H, q, -CH2
CH3), 6.30 (1H, s, olefinic proton) 7.04-7.80
(4H, m, aromatic protons).
Diethyl Oxomalonate 36
This compound was prepared by the method of Dox [30]
, and purified by fractionation under
reduced pressure (103-108 oC/15mm Hg), through
a column (50 cm) packed with Fenske rings. (lit. [30]
103-108 oC/15 mm Hg).
Rearrangement of 3,3-dicarbethoxy-3,4-dihydro-9-(1-methylethylidene)-1,4-methano-1H-2-benzopyran 37 on basic alumina
Purification of the crude diethyl oxomalonate adduct 37,
prepared as above, was also attempted by chromatography on a column
of basic alumina (2 cm dia., 50g, activity III), using mixtures,
of successively increasing polarity, of hexane, ether and methanol
as eluent, but again, none of the adduct 37 was recovered.
This procedure afforded only one compound which appeared to be
related to the adduct 37.
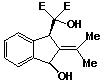
2-(1-methylethylidene)-2,3-dihydro-a,a-dicarbethoxy-syn-1H-inden-3-ol-1-methanol 52 was finally eluted with a mixture of methanol and ether (1:4), and was recovered as a pale yellow coloured oil (0.07 g; 13%).
P.m.r. spectrum (CDCl3): 1.34 (6H, t J = 7 Hz, -OCH2CH3), 1.78 (3H, s, -CH3), 1.94 (3H, s, -CH3), 3.80 (1H, d Ja,b = 10 Hz, exchanged with D2O, Hb), 4.08-4.60 (5H, m, 1H exchanged with D2O, -OCH2CH3 & Hc), 4.90 (1H, d Ja,d = 1 Hz, Hd), 5.38 (1H, dd Ja,b = 10 Hz Ja,d = 10 Hz Ja,d = 1 Hz, Ha), 7.20-7.65 (4H, m, aromatic protons).
Mass spectrum: m/e 348 (M+,
0.01%), 330 (2), 174 (10), 173 (67), 157 (7), 156 (27), 155 (100),
141 (8), 129 (9), 128 (8), 115 (10), all other peaks less than
5%.

3,3-Dicarbethoxy-3,4-dihydro-9-(1-methylethylidene)-1,4-methano-1H-2-benzopyran 37
3,6-Di(2-pyridyl)stetrazine (0.45g; 1.9 mmol)
was added slowly to a stirred solution of the hydrocarbon 33
(0.30g; 1.6 mmol) and diethyl oxomalonate 36(0.25 ml); 1.6 mmol)
in chloroform (10 ml) and the resulting solution stirred for 1
hour. Removal of the solvent afforded the crude product. Attempts
to purify this product by chromatography on silica gel or alumina
only resulted in its rearrangement. However, the p.m.r. spectrum
(CDCl3) of the product could be deduced
from that of the crude compound: d
1.02 (3H, t J = 7 Hz, endo-OCH2CH3),
1.30 (3H, t J = 7 Hz), exo-OCH2CH3),
1.72 (3H, s, methyl protons), 1.76 (3H, s, methyl protons), 3.96
(2H, q J = 7 Hz, endo-OCH2CH3),
4.36 (2H, q J = 7 Hz, exo-OCH2CH3),
4.74 (1H, d Ja,b = 1.5 Hz, Hb)
5.76 (1H, d Ja,b = 1.5 Hz, Ha),
7.10-7.60 (4H, m, aromatic protons), and the mass spectrum
showed the parent ion at m/e 330.
Rearrangement of 3,3-dicarbethoxy-3,4-dihydro-9-(1-methylethylide)-1,4-methano-1H-2-benzopyran 37 on silica gel
Purification of the crude diethyl oxomalonate adduct 37,
prepared as described above, was attempted by preparative thinlayer
chromatography (silica, ether/light petroleum 1:1), but none of
the adduct was recovered. Instead, this procedure afforded two
rearranged products 53 and 54.
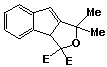
1,1-Dimethyl-3,3(3aH)-dicarbethoxy-1H-indeno[1,2-c] furan 53, less polar band, was recovered as a yellow oil (0.06g; 11%).
infra red spectrum: umax (liquid film) 3550 (m), 3000 (s), 1740 (s), 1610 (m), 1470 (s), 1370 (s), 1300 (s), 1060 (s), 930 (m), 890 (m), 860 (m), 760 (m), 680 (m) cm -1.
P.m.r. spectrum (CDCl3): d 0.82 (3H, t, -OCH2CH3), 1.38 (3H, t, -OCH2CH3), 1.62 (3H, s, -CH3), 1.76 (3H, s, -CH3), 3.60-4.00 (2H, m, -OCH2CH3), 4.20-4.70 (2H, m, -OCH2CH3), 5.00 (1H, d Ja,b = 2 Hz, Hb), 6.58 (1H, d Ja,b = 2 Hz, Ha), 7.08-7.70 (4H, m, aromatic protons).
Mass spectrum (M+, m/e 330.1472. C19H22O5 requires 330.1467);
m/e 331 (7%), 330 (M+, 29), 315(5),
312(30), 258(10), 257(54), 256(8), 243(10), 242(7), 240(10), 239(44),
238(41), 229(5), 212(5), 211(24), 199(6), 198(6), 197(15), 196(15),
186(13), 183(60), 182(6) 172(12), 171(5), 169(8), 168(8), 167(7),
166(7), 157(19), 156(57), 155(45), 154(6), 153(10), 152(7), 143(8),
142(13), 141(60), 140(6), 139(12), 129(20), 128(24), 127(11),
126(7), 116(10), 115(43), 114(6), 99(5), 98(10), 97(12), 96(5),
95(5), 89(8), 85(13), 84(6), 83(39), 82(5), 81(23), 79(5), 77(7),
75(7), 73(7), 71(12), 70(6), 69(16), 67(10), 59(15), 58(9), 57(18),
56(6), 55(44), 53(9), 51(6), 45(15), 44(7), 43(100), 42(10), 41(46),
40(5), 39(24), 32(6), 31(8), 29(68), 28(25), 27(28), all other
peaks less than 5%.
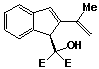
2-(1-Methylethenyl)-a,a-dicarbethoxy-1H-indene-1-methanol 54, more polar band, was also recovered as a yellow oil (0.23g; 44%).
infra red spectrum: umax (liquid film) 3550 (s), 3000 (s), 2250 (w), 1750 (s), 1720 (s), 1665 (m), 1605 (m), 1560 (w), 1470 (s), 1450 (s), 1390 (s), 1370 (s), 1280 (s), 1030 (s), 920 (m), 865 (m), 760 (s), 740 (s) cm-1.
P.m.r. spectrum (CDCl3): d 1.22 (3H, t J = 7 Hz, -OCH2CH3), 1.28 (3H, t J = 7 Hz, -OCH2CH3), 2.06 (3H, s, -CH3), 3.90 (1H, exchanged with D2O, -OH), 4.27 (4H, q J = 7 Hz, -OCH2CH3), 4.76 (1H, s, Hb), 5.08 (2H, s, = CH2), 6.84 (1H, s, Ha), 7.05-7.60 (4H, m, aromatic protons).
Mass spectrum: (M+, m/e 330.1468. C19H22O5 requires 330.1467);
m/e 331 (9%), 330 (M+, 38), 312(10),
257(15), 256(14), 240(8), 239(38), 238(43), 211(15), 184(10),
183(40), 182(10), 171(5), 169(6), 167(6), 166(8), 165(8), 157(14),
156(72), 155(100), 154(16), 153(30), 152(17), 151(6), 143(6),
142(12), 141(47), 140(10), 139(20), 129(22), 128(27), 127(8),
119(6), 116(10), 115(49), 91(5), 89(5), 77(8), 47(9), 45(6), 43(8),
41(5), 31(5), 29(74), 28(8), 27(15), 19(8), 18(50), 17(12), all
other peaks less than 5%.