![]()
![]()
2. Aza-Claisen rearrangement with N-alkenyl-N-benzoylketene-N,O-silyl acetals
2.1. Synthesis of N-alkenyl-N-propionyl-benzamides (2a-e):
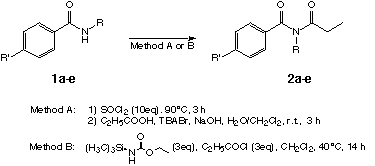
scheme 4
|
Nr. |
R |
R' |
Method |
Yield (%) |
|
2a |
Allyl |
H |
A |
88 |
|
2b |
Crotyl |
H |
B |
93 |
|
2c |
Cyclohexenyl |
H |
B |
93 |
|
2d |
Allyl |
CH3 |
B |
90 |
|
2e |
Allyl |
O-CH3 |
B |
96 |
Methods A and B have been chosen because the starting materials 1a-e were not reactive enough in the presence amine bases [6,7]. Using stronger bases like BuLi lead to side products. Method A, where first an imidoyl chloride is formed and the propionyl rest is then inserted under phase transfer conditions [8], was not applicable for 2c. Therefore another method had to be found. Weinstock [9,10] had reported a synthesis under neutral conditions: As base he used a proton sponge. All other imides 2b-e were synthesized by this simple and efficient method B.
![]()
2.2. Synthesis of N-alkenyl-N-benzoylketene-N,O-tert-butyldimethylsilyl acetals (3a-e):

scheme 5
|
Nr. |
R |
R' |
Yield (%) |
|
3a |
Allyl |
H |
85 |
|
3b |
Crotyl |
H |
93 |
|
3c |
Cyclohexenyl |
H |
90 |
|
3d |
Allyl |
CH3 |
93 |
|
3e |
Allyl |
O-CH3 |
86 |
In contrast to N-butadienyl-N-benzylketene-N,O-silyl acetals [3,4] compounds 3a-e showed high stability towards hydrolysis. So it was possible to purify them by flash chromatography. As expected the introduction of the benzoyl group lead to the desired increased stability. LHMDS proved to be the optimal base in our hands, see also publications Ireland [11] and Ito [12]. The reaction conditions were adapted from the earlier studies in our laboratory [13].
![]()
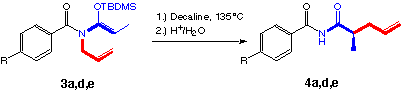
scheme 6
|
Nr. |
R |
Yield (%) |
|
4a |
H |
36 |
|
4d |
CH3 |
49 |
|
4e |
O-CH3 |
41 |
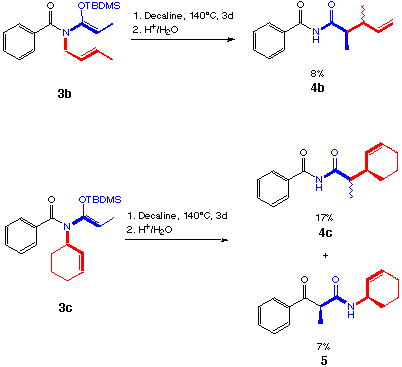
scheme 7
The N-alkenyl-N-benzoylketene-N,O-tert-butyldimethylsilyl acetals (3a,d,e) showed only a moderate aza-Claisen reactivity (scheme 7). Besides the rearranged products 4a,d,e no other product could be identified from the reaction mixture. The thin-layer chromatography indicated a series of degradation products.
The yields from the rearrangement of 3b and 3c were lower than from the rearrangement of 3a,d,e. Mixtures of diastereoisomers were isolated, due to an epimerisation on the Ca after the rearrangement process. With 3c a competing reaction can be observed, the intramolecular acylation (scheme 7).
In literature only a small number of aza-Claisen rearrangements have been reported compared with the number of reports of the Claisen rearrangement [12,14-25]. For the aza-Claisen rearrangement temperatures between 135-148°C are necessary. The best results were obtained, if the formed enolate is directly rearranged in situ without transformation to the TMS- or TBDMS-ether [22]. In view of the planned application in our tandem reaction this method is not useful and was therefore not applied to our substrate.
![]()