Our group has synthesized peptidomimetics
consisting of L-Ala-Gly and Gly-Gly amino acid residues attached to various
stereoisomers of 3,5-dimethyl-Aca linkers. We established through
the use of X-ray crystallography, nuclear magnetic resonance spectroscopy,
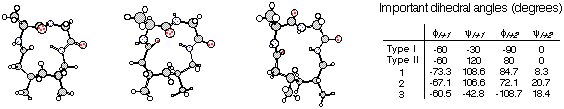
and circular dichroism that the Aca linker stereochemistry induces the
macrocycle to adopt either a type I or a type II b-turn
conformation. Note that the dihedral angles of the induced turns
are in close proximity to idealized values.

Importantly, these were the first molecules of this
class of peptidomimetics to succumb to X-ray crystallographic analysis.
Kitagawa, O.; Vander Velde, D.; Dutta, D.; Morton, M.; Takusagawa,
F.; Aubé, J. J. Am. Chem. Soc. 1995, 117, 5169.
Back to Index
Current Research
