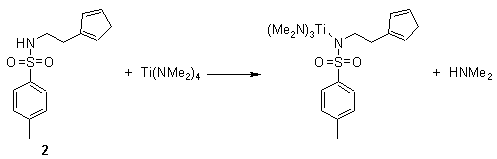
Scheme 6
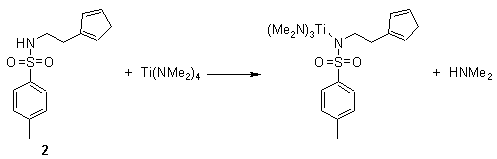
The reaction of the cyclopentadienyl and indenyl ligands 2 and 3 with titanium and zirconium tetradialkylamides in either benzene or toluene proceeds in a stepwise fashion. In the first step the sulfonamide NH reacts with the metal precursor to eliminate one equivalent of dialkylamine. This first step is fast and reaches completion in a few minutes at roomtemperature for all ligands synthesized so far. The reaction is fast for both titanium(tetra) dimethylamide, Ti(NMe2)4, and zirconium(tetra)dimethylamide, Zr(NMe2)4. The first step of the reaction is also fast for the reaction of the ligands 2 and 3a with titanium(tetra)diethylamide Ti(NEt2)4.
Scheme 7
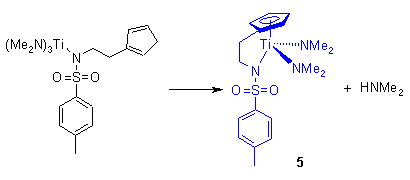
The intermediate product was observed by nmr for the indenyl substituted ligands and for the reaction of ligand 2 with Zr(NMe2)4 and Ti(NEt2)4. It was however not observed for the reaction of 2 with Ti(NMe2)4. This reaction proceeds to completion with the elimination of two equivalents of dimethylamine at roomtemperature to form 5 before the intermediate product could be observed by nmr. Prolonged (days) refluxing in toluene was required for all other reactions to reach completion with the formation of mono nuclear complexes with a bidente cyclopentadienylsulfonamido ligand. However, there was one exception. The reaction of 2 with Zr(NMe2)4 does not proceed cleanly. The expected product can be observed in the nmr spectra but is contaminated with an unidentified mixture of products. Attempts to purify this reaction product have not been successful.
Scheme 8
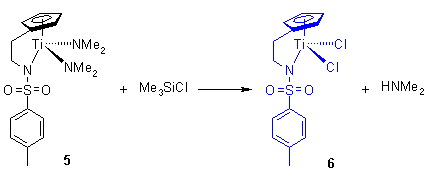
The bis dialkylamide complexes can be converted to the corresponding dichloride by reacting the amides with two equivalents of Me3SiCl in toluene at roomtemperature. The insoluble dichloride complexes precipitate from the reaction mixture and can be isolated analytically pure.
An Xray crystal structure of 6 was determined and can be found here
Experimental details and nmr data can be found below.
Ti(eta5:sigma-C5H4CH2CH2NSO2C6H4CH3)Cl2 (6): To a solution of compound 5 (0.23g, 0.58 mmol) in benzene (4 mL) was added a solution of Me3SiCl (0.13g, 1.20 mmol) in benzene (2 mL). The reaction mixture was stirred overnight at room temperature. The volume was reduced to 50% under reduced pressure. Pentane (10 mL) was added to the reaction mixture and a yellow solid was isolated via filtration. The crude product was washed with a small amount of pentane and dried under vacuo to yield 0.11 g (0.29 mmol, 50 %) of compound 6 as a yellow solid. Anal. Calcd for C14H15Cl2NO2STi: C, 44.2; H, 4.0; N, 3.7. Found: C, 44.5; H, 4.3; N, 4.5. 1H NMR (benzene-d6) d 7.87 (d, 2H, 3JH-H = 8.2 Hz, C6H4), 7.24 (d, 2H, 3JH-H = 8.2 Hz, C6H4), 6.94 (t, 2H, 3JH-H = 2.6 Hz, C5H4), 6.39 (t, 2H, 3JH-H = 2.6 Hz, C5H4), 4.21 (t, 2H, 3JH-H = 7.0 Hz, N-CH2), 3.01 (t, 2H, 3JH-H = 7.0 CH2-C-N), 2.36 (s, 3H, CH3). 13C NMR (benzene-d6) d 148.7 (C6H4 para), 144.9 (C6H4 ipso), 134.4 (C5H4 ipso), 129.8 (CH of C6H4), 128.2 (CH of C6H4), 121.9 (CH of C5H4), 119.4 (CH of C5H4), 64.3 (NCH2), 28.3 (CH2-C-N), 21.6 (CH3).
Ti(eta5:sigma-C9H6CH2CH2NSO2C6H4CH3)(NMe2)2 (7): To a solution of Ti(NMe2)4 (0.68 g, 3.0 mmol) in benzene (5 mL) was added C9H7CH2CH2N(H)SO2C6H4CH3 (0.94 g, 3.0 mmol) as a solid in small portions over a period of 30 minutes. The reaction mixture was refluxed for two days with a slow stream of argon blowing over the top of the reflux condensor to remove liberated dimethylamine. The solvent was removed under reduced pressure. The crude product was subsequently washed with a small amount of pentane to yield compound 3 (1.13 g, 2.52 mmol, 84 %) as a dark orange solid. An analytically pure sample could be obtained by precipitation of a toluene solution with pentane. Anal. Calcd for C22H29N3O2STi: C, 59.1; H, 6.5; N, 9.4. Found: C, 59.1; H, 6.7; N, 9.0. 1H NMR (benzene-d6) d 7.85 (d, 2H, 3JH-H = 8.0 Hz, C6H4), 7.27 (d, 1H, 3JH-H = 7.3 Hz), 7.20 (d, 1H, 3JH-H = 7 Hz), 6.93 (d, 2H, 3JH-H = 8.0 Hz, C6H4), 6.81 (m, 2H), 6.19 (d, 1H, 3JH-H = 3.1 Hz, indenyl-C5), 5.94 (d, 2H, 3JH-H = 3.1 Hz, indenyl-C5), 4.37 (m, 1H, NCH), 3.90 (m, 1H, NCH), 3.45 (s, 6H, NCH3), 2.71 (m, 1H, CH-C-N), 2.48 (s, 6H, NCH3), 2.33 (m, 1H, CH-C-N), 2.02 (s, 3H, CH3). 13C NMR (benzene-d6) not all aromatic signals observed, d 129.1, 125.2, 124.8, 124.7, 122.1, 117.9 (indenyl-C5 CH), 99.6 (indenyl-C5 CH), 60.9 (N-CH2), 51.8 (NMe2), 46.9 (NMe2), 27.6 (CH2-C-N), 22.2 (CH3).
Zr(eta5:sigma-C9H6CH2CH2NSO2C6H4CH3)(NMe2)2 (8): To a solution of Zr(NMe2)4 (1.35 g, 5.1 mmol) in toluene (10 mL) was added a solution of C9H7CH2CH2N(H)SO2C6H4CH3 (1.36 g, 5.1 mmol) in toluene (30 mL). The reaction mixture was refluxed for two days with a slow stream of argon blowing over the top of the reflux condensor to remove liberated dimethylamine. The solvent was removed under reduced pressure. The crude product was subsequently washed with a small amount of pentane to yield compound 8) as a dark orange solid. 1H NMR (benzene-d6) d 7.85 (d, 2H, 3JH-H = 8.1 Hz, C6H4), 7.63 (d, 1H, 3JH-H = 8.4 Hz), 7.55 (d, 1H, 3JH-H = 8.4 Hz), 7.13 (m ,1H), 7.00 (m, 1H), 6.91 (d, 2H, 3JH-H = 8.1 Hz, C6H4), 6.34 (d, 1H, 3JH-H = 3.2 Hz, indenyl-C5), 6.30 (d, 2H, 3JH-H = 3.2 Hz, indenyl-C5), 4.30 (m, 1H, NCH), 3.63 (m, 1H, NCH), 3.19 (s, 6H, NCH3), 3.06 (m, 1H, CH-C-N), 2.78 (m, 1H, CH-C-N), 2.76 (s, 6H, NCH3), 2.01 (s, 3H, CH3). 13C NMR (benzene-d6) d 142.6, 138.7, 129.4, 125.7, 121.4, 129.7 (CH), 127.6 (CH), 124.2 (CH), 124.0 (CH), 123.9 (CH), 120.5 (CH), 116.8 (indenyl-C5 CH), 94.7 (indenyl-C5 CH), 52.3 (N-CH2), 46.4 (NMe2), 42.7 (NMe2), 27.7 (CH2-C-N), 21.2 (CH3).