

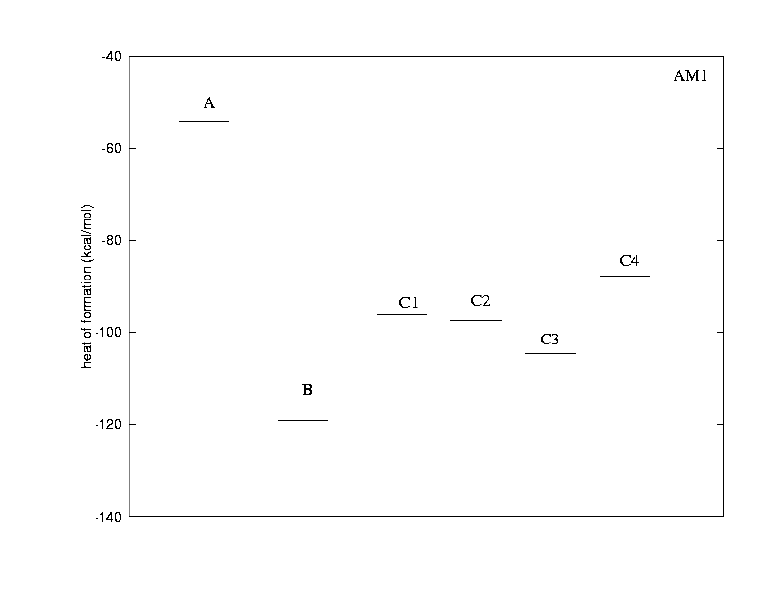
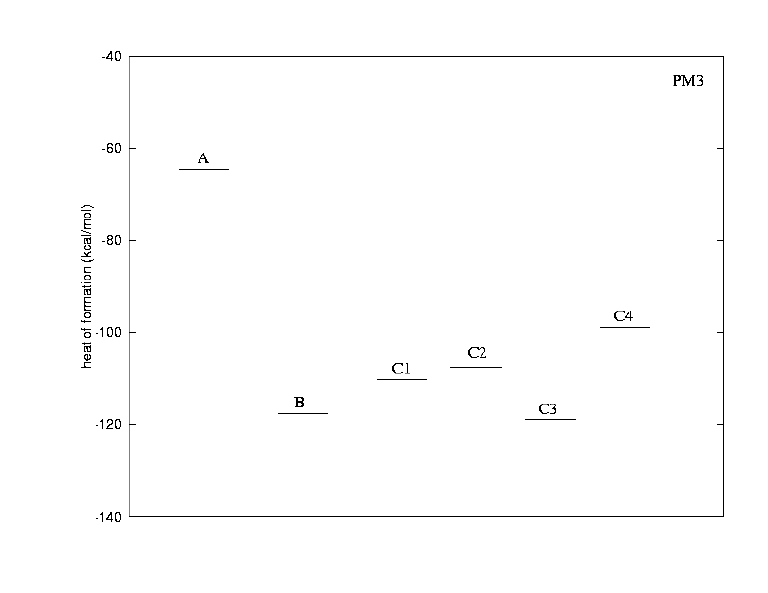
The calculations were done to clarify the following points:
Generally, the ring closure of the two C-C triple bonds is energetically highly favoured; going from A to B lowers the energy by 65 kcal/mol (AM1) or 53 kcal/mol (PM3). This suggests that ring strain in the products is not important for the energetics of the reaction. Due to limitations of the calculations by the size of the molecules, one cannot use ab initio methods to explore how platinum lowers the energy barrier for the reaction.
From the experimental data, we know that isomer B is not formed, but one of the isomeric structures C. Interestingly, the AM1 calculation suggests that B lies lower in energy than all of the C isomers, while PM3 attributes a very similar energy to isomer C3. From a purely thermodynamical point of view, B might therefore be an intermediate in the formation of only C3. This can be excluded, however, for geometric reasons, since there is no sterically reasonable way how the oxygen could be transferred back from the carbonyl carbon to sulphur.
Isomer C3 appears to be the most stable compound of the series according to both AM1 and PM3 results. A prediction which isomer is obtained experimentally is not possible, as we cannot say if the reaction is kinetically or thermodynamically controlled. For comparison, we show the most (C3,left) and the least (4,right) stable of the isomeric structures. Both similarities (preferably in the center) and differences (mainly in the periphery) can be seen clearly. The difference in energy is mainly due to steric reasons.
 |
 |