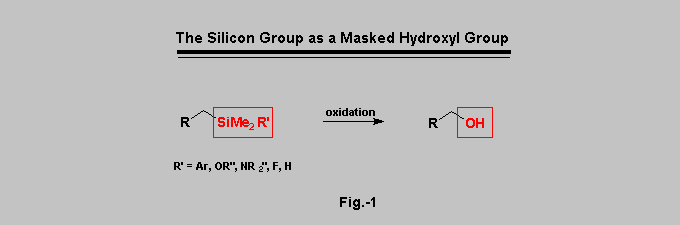
Since its discovery independently by Tamao-Kumada and by Fleming [1][2][3],
the oxidation of the carbon-silicon bond to the corresponding
carbon-hydroxyl bond has become a common and widely employed tool in
organic synthesis (Fig.-1). The use of a masked hydroxyl group is indeed
very convenient and avoids the sometimes troublesome protection-
deprotection sequence required by the presence of a free hydroxyl group.
ArMe2Si and (RO)Me2Si groups are among the most versatile masked hydroxyl
groups but they also present some drawbacks [1][2].
The PhMe2Si group :
- It is the most frequently used in multistep synthesis due to its high
stability, [4].
- However, it requires an acidic or an electrophilic treatment before the
oxidation, which in certain cases may not be compatible with other
functionalities [5] .
The (RO)Me2Si group :
- the Si-O bond is relatively fragile,
- but, it is easily oxidized using mild conditions, compatible with most
functionalities [1].


