Application to benzyl and allylsilanes
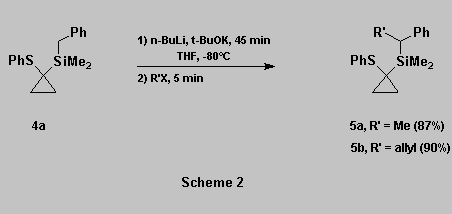
To test the viability of our
methodology, we prepared the benzylsilane 4a
and allylsilane 4b and submitted them to
metallation conditions using Schlosser's superbase [9].
Alkylation and allylations of 4a occurred smoothly to afford
5a-b in good yields (Scheme 2).
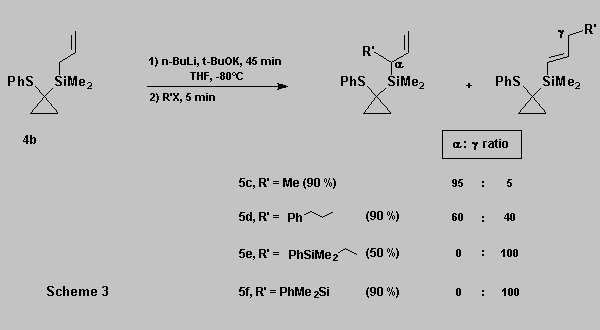
As expected, the regioselectivity during the alkylation of
allylsilane 4b is electrophile dependent [10] (Scheme 3).
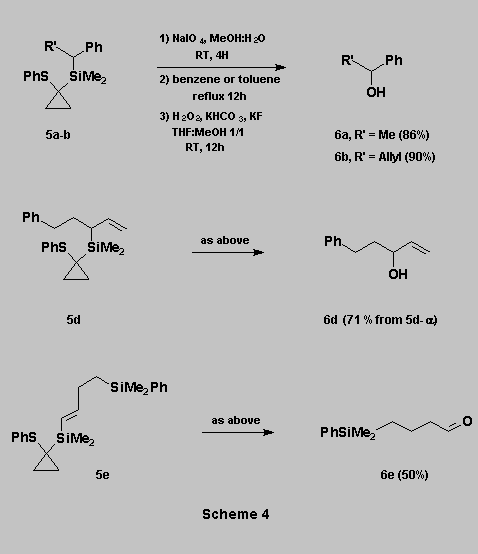
Oxidation of the C-Si bond
Oxidation of 5
to sulfoxides using NaIO4 in MeOH-H2O, followed by thermal
rearrangement in benzene (or toluene for 5e) under reflux,
afforded the corresponding siloxanes in quantitative yields. These were
then subjected to Tamao oxidation [1] to give the expected alcohols 6a-e.
* The three step oxidation sequence can be carried out
without purification of any of the intermediates.
* An allylic double bond is not affected by the reaction conditions, allowing a mild conversion of an allylsilane (i.e. 5d) into the corresponding allylic alcohol (i.e. 6d)
* An aldehyde function can also be obtained from the corresponding vinylsilane (i.e. 6e).
* The PhMe2Si group is not affected by the oxidative conditions (i.e. 6e) (Scheme 4).