A recent
report by Abd. Rahman and Fleming showed that oxidative cleavage of a C-Si bond directly attached to a
cyclopropane ring is not possible using the PhMe2Si group
[16]. The electrophilic conditions used in this occasion led to the opening of the cyclopropane ring.
We demonstrate here that our methodology affords an
alternative route to produce the desired cyclopropyl alcohol.
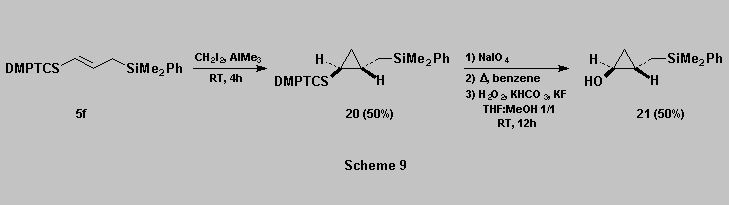
Cyclopropanation of allylsilane 5f using a modification of
the Simmons-Smith reaction [17a] gave the
expected cyclopropane 20 [18] which was then oxidized, with retention of configuration, to afford the
cyclopropanol 21 (Scheme
9).

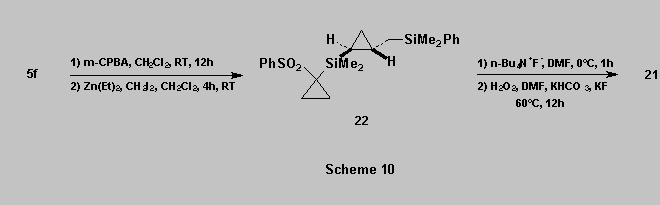
Similarly, oxidation of 5f into the corresponding
sulfone followed by cyclopropanation [17b] gave
the cyclopropane 22 which was then oxidized using a two step sequence :
*
displacement of the cyclopropylsulfone with a fluoride source, to form the Si-F bond,
*
oxidation of the resulting SiMe2F group, giving 21 in 30%
overall yield (from 5f) [3d][19][20].