Calculations of the Second Order Hyperpolarizabilities (beta) of Some New Compounds
In order to estimate the potential of our new compounds for applications in the NLO field,
we have carried out preliminary calculations with the ZINDO method [9],
using the HyperChem software package. Standard parametrizations were
used for the geometry optimizations of 3 and 8a. The UV/VIS spectra were
calculated with a sigma-sigma overlap factor of 1.267 and a pi-pi overlap factor of 0.585,
including 11 occupied and 11 unoccupied orbitals in the configuration interaction.
Calculated transitions in the visible region for 3 and 8a
(nm; oscillator strength in brackets), and the corresponding observed values in acetone
(nm, epsilon):
3 (calc.) 3 (obs.) 8a (calc.) 8a (obs.)
611 (0.0056) 691 (4190) 578 (0.0214) 502 (2870)
550 (0.0024) 369 (0.3125) 396 (8720)
493 (0.0995) 323 (1.6381) 334 (16000)
428 (0.0070) 439 (11400) 332 (12000)
425 (0.0066) 432 (11400)
379 (0.0121)
372 (0.0233)
366 (0.0013)
351 (0.0473)
335 (0.0508) 332 (8140)
As observed for other ferrocene derivatives [10],
calculated and observed transitions are in modest agreement; particularily for
the cation 3, strong solvation effects have to be considered which
make it difficult to compare the values.
As mainly the metal-to-ligand transitions are responsible for the NLO properties
of ferrocene derivatives and the main contribution to the beta value can be attributed to a
single transition
[10], we can connect the linear optical properties with the non-linear ones
by the two-level Oudar model
[11].
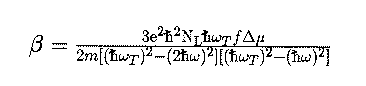
Here, omega denotes the excitation wavelength and omegaT the transition under
investigation.
In the preliminary calculations, no attempts have been made to verify this very simple
model for these special cases, and tentative beta values have been calculated
for a few transitions
in the visible region only (UV transitions seem to be less important for the hyperpolarizability)
[10].
For an excitation wavelength of 1024 nm, the calculated beta-values for 3 are:
611 nm: beta= 178,600; 425 nm: beta= 79,970; 372 nm: beta= 1,012,000; 335 nm: 751,900;
and for 8a : 578 nm: beta = 303,000; 369 nm: beta= 4,144,000; 323 nm: beta= 19,140,000
A3s3m3/J2, respectively.
This means that the NLO properties of the alkene should be at least similar to that of known
ferrocene derivatives with good NLO properties (e.g., 1-ferrocenyl-2-(p-nitrophenyl)ethene,
beta = 10,500,000 A3s3m3/J2 for a
typical metal-to-ligand transition at 367 nm).
R.Herrmann, 25th May 1995