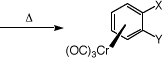Introduction
The exploitation of the modification of reactivity of an organic molecule by complexation to a
metal is one of the major areas of development in organic synthesis in recent times1. One particular
area is the study of the enhanced reactivity of arenes upon complexation by Group VI metal and
manganese carbonyls. Among these by far the most studied are the arenechromiumtricarbonyl
complexes2. The effect of the metal moiety on the arene ring is an apparent electron withdrawal
from the p-system and this manifests itself in a variety of ways as shown below:
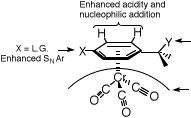 Y = H,Enhanced acidity
Y = H,Enhanced acidity
Y = L.G., Enhanced solvolysis
Y = (+) or (-),Stabilised
Stereocontrol
It is important for the ease of use of such complexes that they should be readily prepared and
efficiently decomplexed. This experiment demonstrates the most convenient method of preparation
viathe use of the Strohmeier apparatus3 and a simple vacuum line/nitrogen manifold (see
Appendix). The complexes are synthesised by the direct reaction between the arene and chromium
hexacarbonyl in an ether solvent mixture (Bu2O-THF 10-1)4.
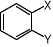 +Cr(CO)6
+Cr(CO)6
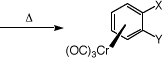 The need for the Schlenk line arises from the fact that the intermediates generated during the
synthesis, the coordinatively unsaturated chromium carbonyl species, are very oxygen sensitive and
rigorously anaerobic conditions are essential. Once formed the arene complexes are air stable in
the solid state and can be handled without difficulty by conventional techniques.
The Strohmeier apparatus is, in effect, an inverted condenser. This is a convenient way of dealing
with the problem of the volatility of the metal carbonyl which tends to condense above the level of
the solvent in a normal condenser and block it. In the Strohmeier apparatus, any hexacarbonyl
condensing ahead of the solvent is washed back into the reaction vessel viathe syphon.
The need for the Schlenk line arises from the fact that the intermediates generated during the
synthesis, the coordinatively unsaturated chromium carbonyl species, are very oxygen sensitive and
rigorously anaerobic conditions are essential. Once formed the arene complexes are air stable in
the solid state and can be handled without difficulty by conventional techniques.
The Strohmeier apparatus is, in effect, an inverted condenser. This is a convenient way of dealing
with the problem of the volatility of the metal carbonyl which tends to condense above the level of
the solvent in a normal condenser and block it. In the Strohmeier apparatus, any hexacarbonyl
condensing ahead of the solvent is washed back into the reaction vessel viathe syphon.





 1
2
3
4
5
1
2
3
4
5

![]()
![]()
![]()

![]()