
|
Density |
1.65 g cm-3 | |||||||
|---|---|---|---|---|---|---|---|---|
|
Standard heat of formation |
9.08 k cal mol-1 | |||||||
|
Index of refraction |
2.2 (600nm) | |||||||
|
Boiling point |
Sublimes at 800K | |||||||
|
Resistivity |
1014 ohms m-1 | |||||||
|
Vapour density |
N/A | |||||||
|
Crystal form |
Hexagonal cubic | |||||||
|
Vapour pressure |
5 × 10-6 torr at room temperature | |||||||
|
Organoleptic properties |
Appearance |
| ||||||
|
Odour |
Odourless | |||||||
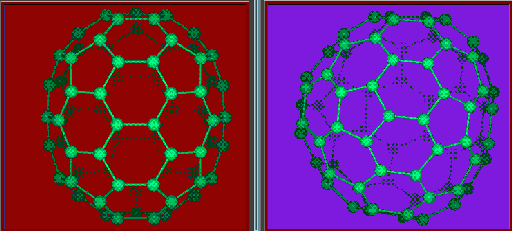
You can find more discussion about the structure of C60 Here
![]()
Fullerene structures possess many symmetries. This is because the molecules are round. More informations such as bond lengths, radii of the molecules can be view here
Reactivities of fullerenes
The most exciting characteristic of the buckyball is that it is hollow on the inside, and some elements in the periodic table will fit inside. This creates the main reactivity fo fullerenes.
Fullerenes reacts with many elements. This includes metallic element and non-metallic elements. Example are the reaction of fullerenes with Noble Gas and the reaction of fullerenes with metal. Fullerenes also react with metals to form Metallocarbohedrenes.