Introduction
Since its approval nearly 20 years ago by the FDA, Salbutamol (known as Albuterol in the United States of America) has become the life-saving drug of choice for the millions of people worldwide afflicted with asthma. At the time of its development in 1970, Salbutamol represented a quantum leap in asthma therapy. Like existing drugs, it functioned on the basis of inducing bronchodilation through stimulation of beta2 receptors on the cells lining the airways in the lungs; unlike existing drugs, however, Salbutamol could be delivered in small enough doses to only interact with beta2 receptors whilst still being effective.
Existing treatments such as Bronkosol (Isoetharine) had both beta1 and beta2 effects, resulting in side-effects such as increased heart rate, palpitations, tremor, and nausea. Furthermore, a number of these older bronchodilators had additives which increased sensitivities while doing nothing to increase effectiveness, such as saccharin, now known to be a carcinogen. Another common drawback to many earlier bronchodilators was their short duration of action, usually 1-4 hours. Salbutamol extended this to six hours.
Salbutamol is marketed under a number of names including Ventolin, Proventil, Albuterol, Aerolin, Salbulin, Ventodisks, Asmol, Respax and Respolin. However, these names encompass both salbutamol and its sulphate salt, a form in which it is commonly delivered.
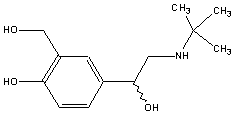
2-(tert-butylamino)-1-(4-hydroxy-3-hydroxymethylphenyl) ethanol –

Salbutamol
2-(tert-butylamino)-1-(4-hydroxy-3-hydroxymethylphenyl) ethanol hemisulfate
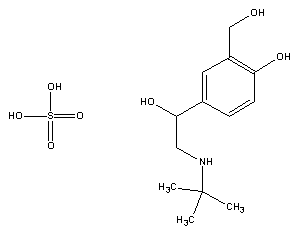
Salbutamol Sulphate