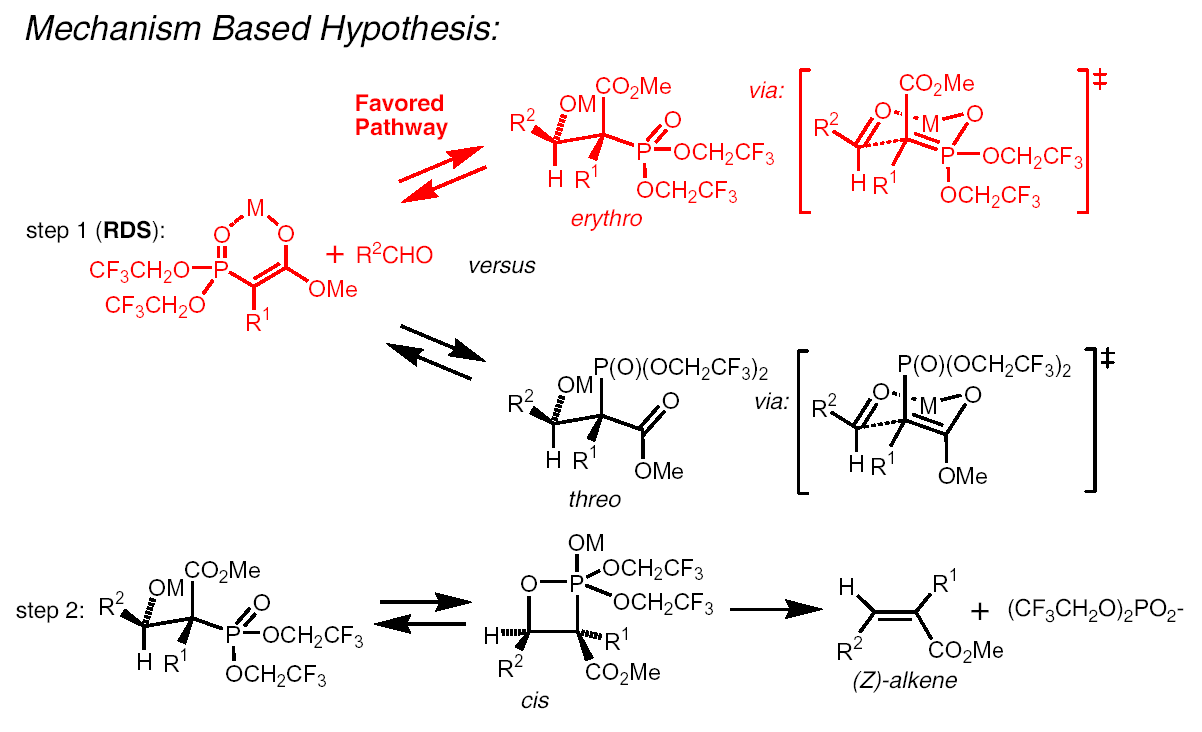
(Z)-stereospecific Olefination (Wittig reaction analogue)
Utilising Still-Gernnari reagent

Why is this reaction stereospecific?
The 'erythro' adduct is more favourable than the 'threo' adduct
as the transition state is the less hindered, therefore thermodynamically stable,
thus in step 2, the (Z)-alkene is formed:
Journal: Still, W.C.; Gennari, C., TETRAHEDRON LETTERS, 1983,
24, 4405


