n-Butyl lithium is hexameric in the solid state[1] and in cyclohexane solutions. Why? Here I try to find out some of its secrets.
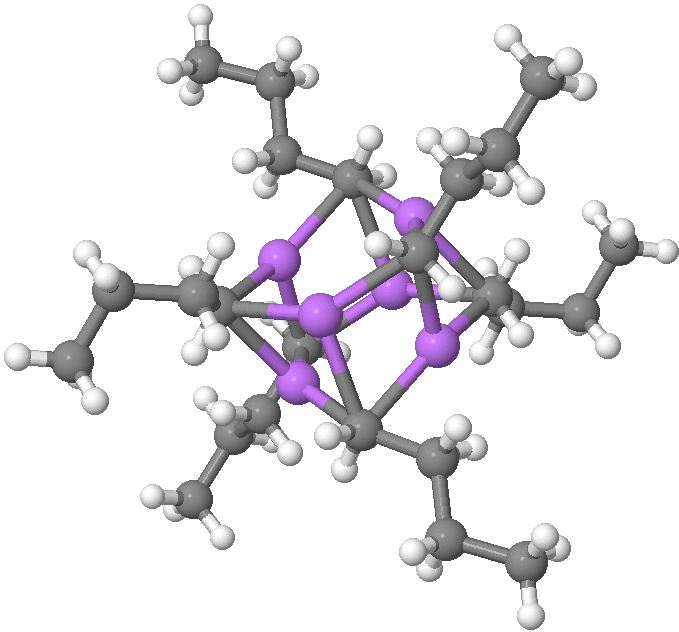
SUHBEC. CLICK FOR 3D.
The crystal structure reveals the following points of interest:
- Six lithium atoms form a cluster with triangular faces.
- An off-centre carbanion caps a triangular lithium face.
- Four of the butyl groups are in a fully extended antiperiplanar conformation
- But two di-axial n-butyl exhibit a gauche conformation.
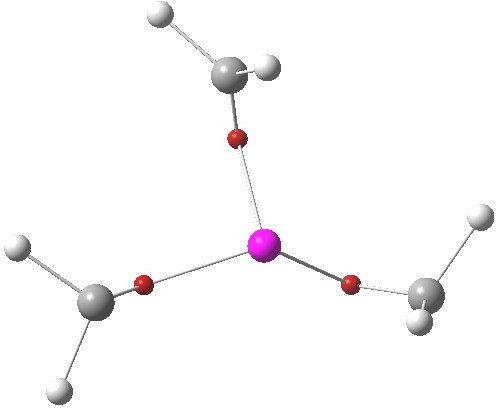
The lithium cluster has twelve electrons available for bonding; if the Li is considered as Li+, balanced by six C– carbanions, the twelve electrons come from the six carbon lone pairs pointing towards each of six triangular faces. An ELF analysis can help identify how these twelve electrons are arranged. Shown below is the environment of a single Li-face, with the ELF basin ringed. It integrates to 2.08 electrons. So each tetrahedral cluster of three lithiums and one carbanion could be considered as a two-electron-four-centre bond, perhaps a natural progression from the two-electron-three-centre bonding found in a slightly less electron deficient system such as diborane.
ELF basins. Click for 3D
NBOs (natural bond orbitals) reflect this character. An NBO represents a localised two-electron orbital, and analysis indeed reveals six such orbitals, each having the form shown below.
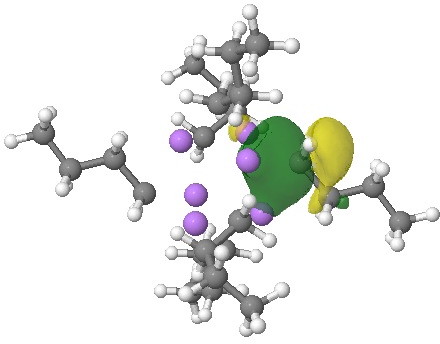
NBO. Click for 3D.
This picture in turn leads us to identify this system as spherically aromatic[2]. The three-dimensional equivalent of the Hückel rule is that any system with 2(N+1)2 σ or π electrons (or both) in a cluster can be considered aromatic/diatropic. In this case, N=0 and hence the magic count is 2 for each of the six CLi3 tetrahedra. The diatropic ring current might be manifested in the computed 1H NMR chemical shifts of the CH2– protons (-0.8ppm). Aromaticity does not immediately spring to mind with the name n-butyl lithium, but this unprepossessing molecule has six aromatic regions!
Each lithium atom is in turn hemispherically surrounded by three of these 2.08 electron basins (below, although the ELF centroid is very much biased towards the carbon, indicating considerable ionicity). What wonderful electronic economy! Despite there being only twelve electrons to be shared amongst six lithium atoms, each lithium manages nevertheless to surround itself with 6.24 electrons. All crammed into one half sphere, leaving a nice coordination hole; n-butyl lithium is after all a highly reactive species (even as a hexamer).
I want to finish by exploring the observation that two of the six n-butyl groups adopt a gauche conformation. In free n-butane itself, around 31% of the population adopts this shape, which curiously is around the same proportion as is found in the hexameric structure of n-butyl lithium. More generally, a search of the Cambridge database for compounds containing such groups reveals the following distribution; about 1 in 7.
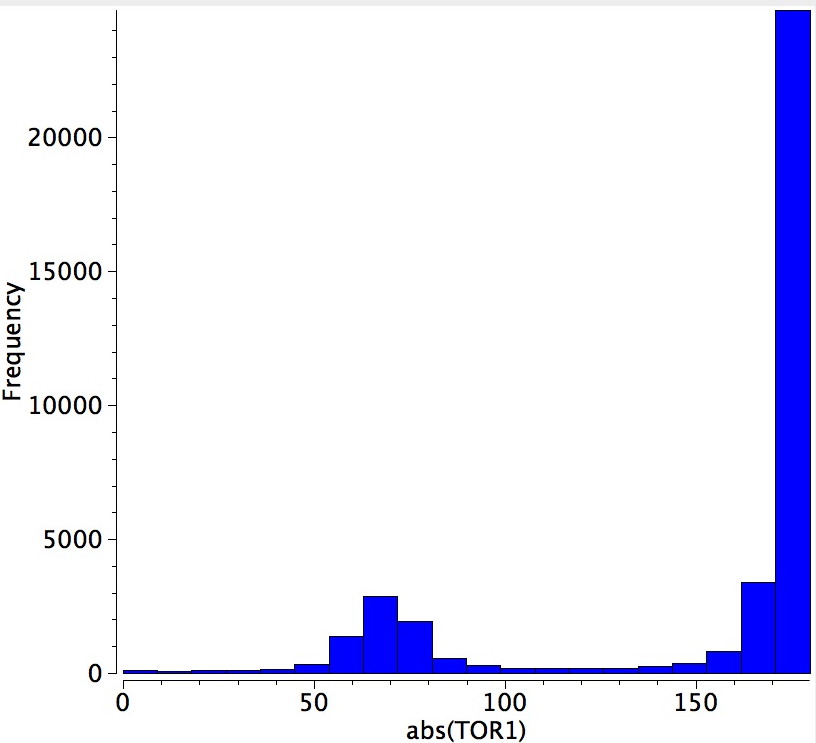
Well, when you deprive a molecule of electrons, as any species with lithium must invariably suffer from, it is wonderful how the system responds. In this sense, a hexameric structure seems a very natural outcome. And it has brought us the two-electron-four-centre bond and the associated spherical aromaticity, both of which are a nice bonus.
References
- T. Kottke, and D. Stalke, "Structures of Classical Reagents in Chemical Synthesis: (<i>n</i>BuLi)<sub>6</sub>, (<i>t</i>BuLi)<sub>4</sub>, and the Metastable (<i>t</i>BuLi · Et<sub>2</sub>O)<sub>2</sub>", Angewandte Chemie International Edition in English, vol. 32, pp. 580-582, 1993. https://doi.org/10.1002/anie.199305801
- A. Hirsch, Z. Chen, and H. Jiao, "Spherical Aromaticity inIh Symmetrical Fullerenes: The 2(N+1)2 Rule", Angewandte Chemie, vol. 39, pp. 3915-3917, 2000. https://doi.org/10.1002/1521-3773(20001103)39:21<3915::aid-anie3915>3.0.co;2-o
Tags: Cambridge, chemical shifts, conformational analysis, cyclohexane solutions, hexameric, spherical aromaticity, Tutorial material