The Bürgi–Dunitz angle describes the trajectory of an approaching nucleophile towards the carbon atom of a carbonyl group. A colleague recently came to my office to ask about the inverse, that is what angle would an electrophile approach (an amide)? Thus it might approach either syn or anti with respect to the nitrogen, which is a feature not found with nucleophilic attack. My first thought was to calculate the wavefunction and identify the location and energy (= electrophilicity) of the lone pairs (the presumed attractor of an electrophile). But a better more direct approach soon dawned. A search of the crystal structure database. Here is the search definition, with the C=O-E angle, the O-E distance and the N-C=O-E torsion defined (also specified for R factor < 5%, no errors and no disorder).
 The first plot is of the torsion vs the distance, for E = H-X (X=O,F, Cl)
The first plot is of the torsion vs the distance, for E = H-X (X=O,F, Cl) 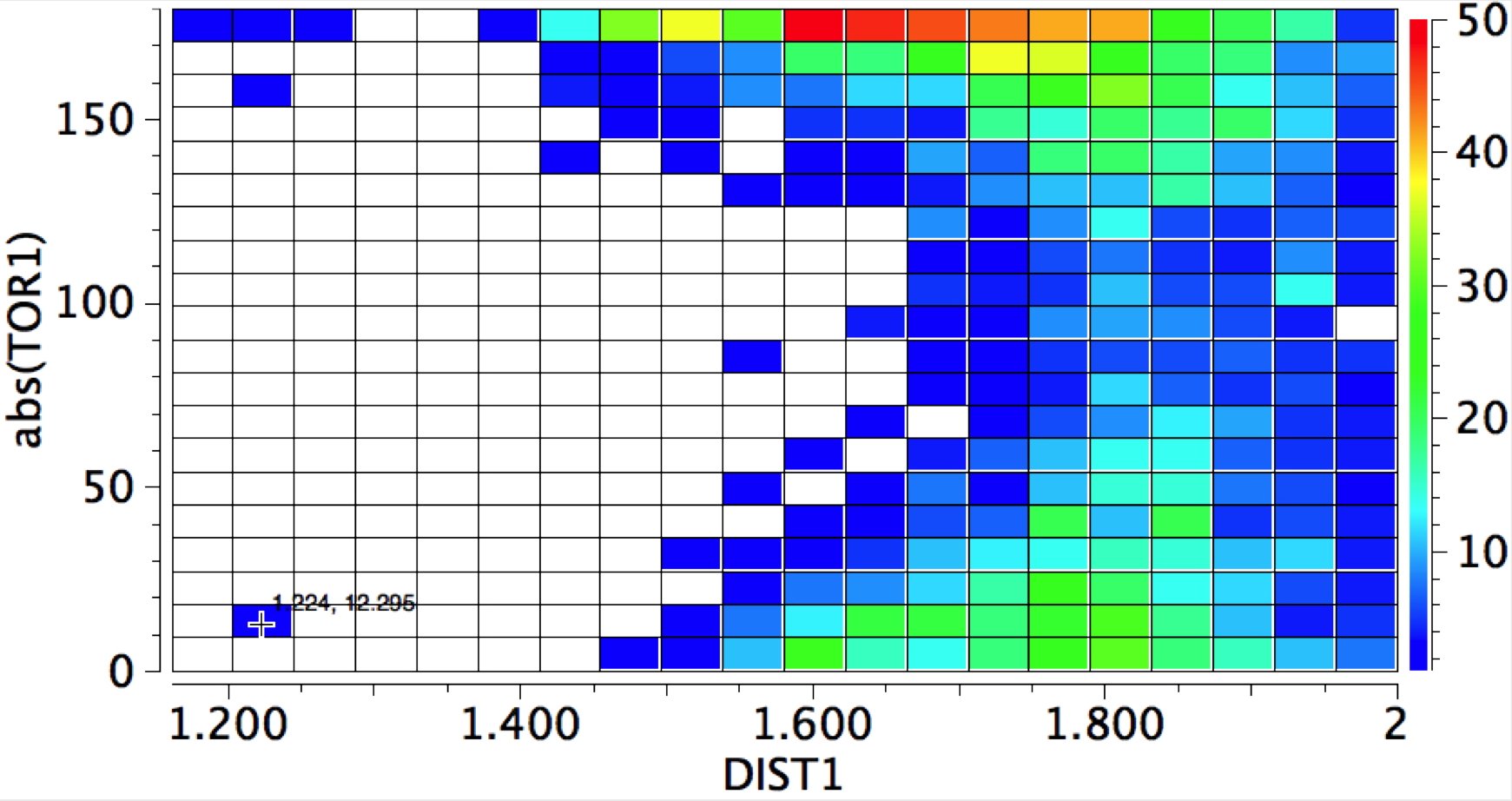
Archive for the ‘General’ Category
Anchoring chemistry.
Wednesday, June 18th, 2014I was reminded of this article by Michelle Francl[1], where she poses the question “What anchor values would most benefit students as they seek to hone their chemical intuition?” She gives as common examples: room temperature is 298.17K (actually 300K, but perhaps her climate is warmer than that of the UK!), the length of a carbon-carbon single bond, the atomic masses of the more common elements.
References
- M. Francl, "Take a number", Nature Chemistry, vol. 5, pp. 725-726, 2013. https://doi.org/10.1038/nchem.1733
Trigonal bipyramidal or square pyramidal: Another ten minute exploration.
Friday, May 2nd, 2014This is rather cranking the handle, but taking my previous post and altering the search definition of the crystal structure database from 4- to 5-coordinate metals, one gets the following.
What is the best way of folding a straight chain alkane?
Sunday, April 6th, 2014In the previous post, I showed how modelling of unbranched alkenes depended on dispersion forces. When these are included, a bent (single-hairpin) form of C58H118 becomes lower in free energy than the fully extended linear form. Here I try to optimise these dispersion forces by adding further folds to see what happens.
Modelling the geometry of unbranched alkanes.
Saturday, March 29th, 2014By about C17H36, the geometry of “cold-isolated” unbranched saturated alkenes is supposed not to contain any fully anti-periplanar conformations. [1] Indeed, a (co-crystal) of C16H34 shows it to have two-gauche bends.[2]. Surprisingly, the longest linear alkane I was able to find a crystal structure for, C28H58 appears to be fully extended[3],[4] (an early report of a low quality structure for C36H74[5] also appears to show it as linear).‡ Here I explore how standard DFT theories cope with these structures.
References
- N.O.B. Lüttschwager, T.N. Wassermann, R.A. Mata, and M.A. Suhm, "The Last Globally Stable Extended Alkane", Angewandte Chemie International Edition, vol. 52, pp. 463-466, 2012. https://doi.org/10.1002/anie.201202894
- N. Cocherel, C. Poriel, J. Rault‐Berthelot, F. Barrière, N. Audebrand, A. Slawin, and L. Vignau, "New 3π‐2Spiro Ladder‐Type Phenylene Materials: Synthesis, Physicochemical Properties and Applications in OLEDs", Chemistry – A European Journal, vol. 14, pp. 11328-11342, 2008. https://doi.org/10.1002/chem.200801428
- S.C. Nyburg, and A.R. Gerson, "Crystallography of the even <i>n</i>-alkanes: structure of C<sub>20</sub>H<sub>42</sub>", Acta Crystallographica Section B Structural Science, vol. 48, pp. 103-106, 1992. https://doi.org/10.1107/s0108768191011059
- R. Boistelle, B. Simon, and G. Pèpe, "Polytypic structures of n-C28H58 (octacosane) and n-C36H74 (hexatriacontane)", Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry, vol. 32, pp. 1240-1243, 1976. https://doi.org/10.1107/s0567740876005025
- H.M.M. Shearer, and V. Vand, "The crystal structure of the monoclinic form of n-hexatriacontant", Acta Crystallographica, vol. 9, pp. 379-384, 1956. https://doi.org/10.1107/s0365110x5600111x
Happy 25th birthday, WWW.
Wednesday, March 12th, 2014A short post this, to remind that today is officially the 25th birthday of the World-Wide-Web, in March 1989. It took five years for a conference around the theme to be organised and below is a photo from that event.
A congruence of concepts: conformations, configurations, amides and enzymes
Sunday, February 9th, 2014This is the time of year when I deliver two back-2-back lecture courses, and yes I do update and revise the content! I am always on the look-out for nice new examples that illustrate how concepts and patterns in chemistry can be joined up to tell a good story. My attention is currently on conformational analysis; and here is an interesting new story to tell about it.
3D-rendered molecular models on this blog: an update.
Thursday, January 16th, 2014So much to do, so little time to do it. That is my excuse at least. Right from my first post on this blog in 2008 I have tried to enhance it using Jmol, a Java-based applet (normally indicated with the caption Click for 3D). This has been pretty stable for some five years now, but a recent spate of security-based releases of the JRE (Java runtime environment) for desktop computers has impacted, the latest of which was released yesterday (Java 7, V 51). Put simply, when I started, an unsigned applet was fine. Now to run, it can only be a properly signed applet. Fortunately, there are two solutions:
Refactoring my lecture notes on pericyclic reactions.
Sunday, December 29th, 2013When I first started giving lectures to students, it was the students themselves that acted as human photocopiers, faithfully trying to duplicate what I was embossing on the lecture theatre blackboard with chalk. How times have changed! Here I thought I might summarise my latest efforts to refactor the material I deliver in one lecture course on pericyclic reactions (and because my notes have always been open, you can view them yourself if you wish).