Ken Houk’s group has recently published this study of cycloaddition reactions, using a combination of classical transition state location followed by molecular dynamics trajectory calculations,[cite]10.1021/jacs.8b12674[/cite] and to which Steve Bachrach’s blog alerted me. The reaction struck me as being quite polar (with cyano groups) and so I took a look at the article to see what both the original[cite]10.1021/jo00042a039[/cite] experimental conditions were and how the new simulations compared. The reaction itself is shown below.
Archive for the ‘reaction mechanism’ Category
An Ambimodal Trispericyclic Transition State: the effect of solvation?
Thursday, May 2nd, 2019Smoke and mirrors. All is not what it seems with this Sn2 reaction!
Thursday, April 4th, 2019Previously, I explored the Graham reaction to form a diazirine. The second phase of the reaction involved an Sn2′ displacement of N-Cl forming C-Cl. Here I ask how facile the simpler displacement of C-Cl by another chlorine might be and whether the mechanism is Sn2 or the alternative Sn1. The reason for posing this question is that as an Sn1 reaction, simply ionizing off the chlorine to form a diazacyclopropenium cation might be a very easy process. Why? Because the resulting cation is analogous to the cyclopropenium cation, famously proposed by Breslow as the first example of a 4n+2 aromatic ring for which the value of n is zero and not 1 as for benzene.[cite]10.1021/ja01576a067[/cite] Another example of a famous “Sn1” reaction is the solvolysis of t-butyl chloride to form the very stable tertiary carbocation and chloride anion (except in fact that it is not an Sn1 reaction but an Sn2 one!)
Free energy relationships and their linearity: a test example.
Sunday, January 13th, 2019Linear free energy relationships (LFER) are associated with the dawn of physical organic chemistry in the late 1930s and its objectives in understanding chemical reactivity as measured by reaction rates and equilibria.
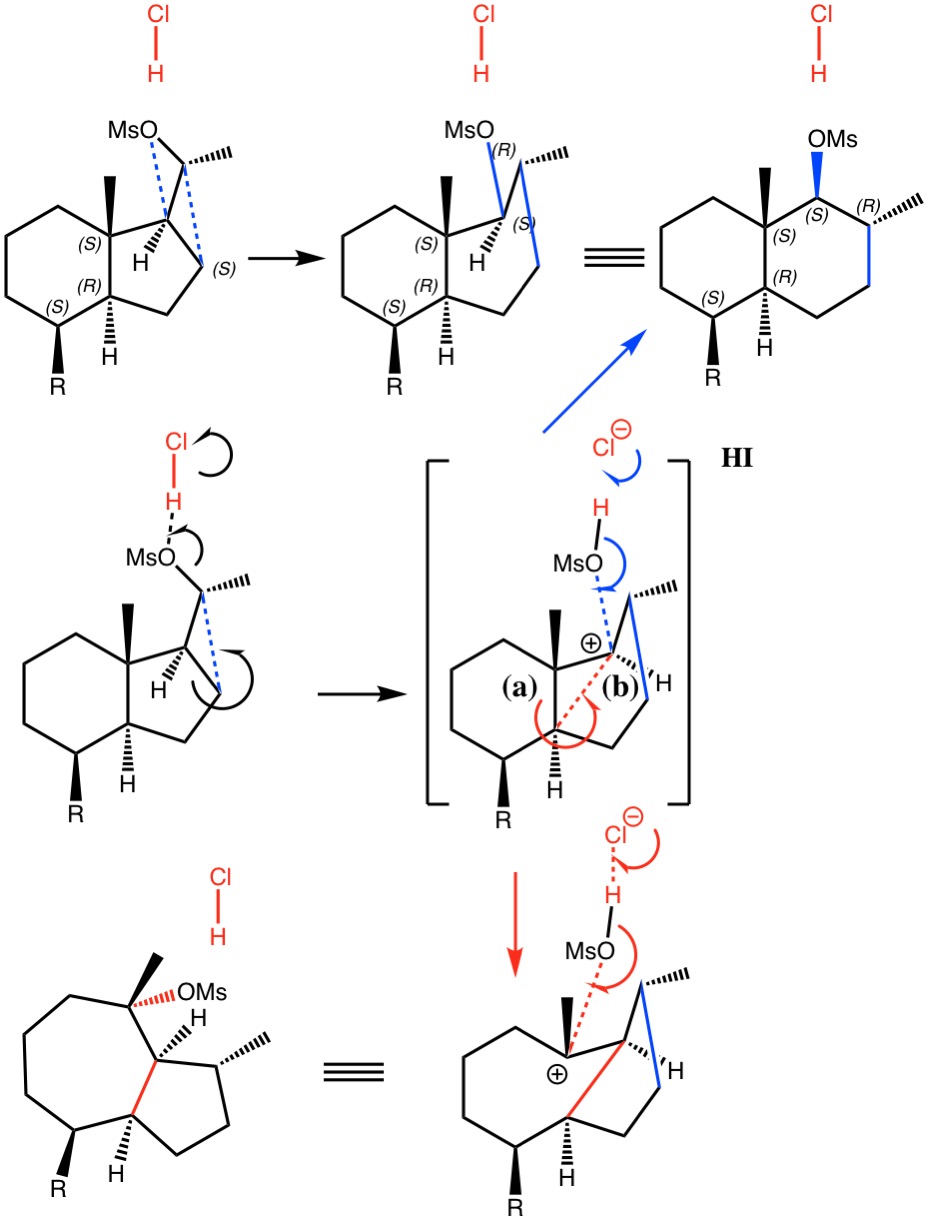
Dyotropic Ring Expansion: more mechanistic reality checks.
Sunday, October 1st, 2017I noted in my WATOC conference report a presentation describing the use of calculated reaction barriers (and derived rate constants) as mechanistic reality checks. Computations, it was claimed, have now reached a level of accuracy whereby a barrier calculated as being 6 kcal/mol too high can start ringing mechanistic alarm bells. So when I came across this article[cite]10.1021/acs.orglett.7b01621[/cite] in which calculated barriers for a dyotropic ring expansion observed under mild conditions in dichloromethane as solvent were used to make mechanistic inferences, I decided to explore the mechanism a bit further.
The conformation of enols: revealed and explained.
Thursday, April 6th, 2017Enols are simple compounds with an OH group as a substituent on a C=C double bond and with a very distinct conformational preference for the OH group. Here I take a look at this preference as revealed by crystal structures, with the theoretical explanation.
What is the (calculated) structure of a norbornyl cation anion-pair in water?
Saturday, April 1st, 2017In a comment appended to an earlier post, I mused about the magnitude of the force constant relating to the interconversion between a classical and a non-classical structure for the norbornyl cation. Most calculations indicate the force constant for an “isolated” symmetrical cation is +ve, which means it is a true minimum and not a transition state for a [1,2] shift. The latter would have been required if the species equilibrated between two classical carbocations. I then pondered what might happen to both the magnitude and the sign of this force constant if various layers of solvation and eventually a counter-ion were to be applied to the molecule, so that a bridge of sorts between the different states of solid crystals, superacid and aqueous solutions might be built.
Reaction coordinates vs Dynamic trajectories as illustrated by an example reaction mechanism.
Monday, March 20th, 2017The example a few posts back of how methane might invert its configuration by transposing two hydrogen atoms illustrated the reaction mechanism by locating a transition state and following it down in energy using an intrinsic reaction coordinate (IRC). Here I explore an alternative method based instead on computing a molecular dynamics trajectory (MD).
How does silane invert (its configuration)?
Thursday, March 16th, 2017In the previous post, I found intriguing the mechanism by which methane (CH4) inverts by transposing two of its hydrogens. Here I take a look at silane, SiH4.