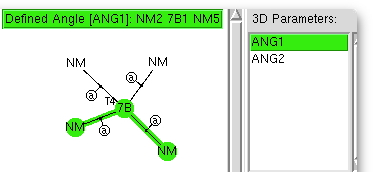
This is rather cranking the handle, but taking my previous post and altering the search definition of the crystal structure database from 4- to 5-coordinate metals, one gets the following.
Archive for the ‘crystal_structure_mining’ Category
Trigonal bipyramidal or square pyramidal: Another ten minute exploration.
Friday, May 2nd, 2014Artemisinin: are stereo-electronics at the core of its (re)activity?
Sunday, April 13th, 2014Around 100 tons of the potent antimalarial artemisinin is produced annually; a remarkable quantity given its very unusual and fragile looking molecular structure (below). When I looked at this, I was immediately struck by a thought: surely this is a classic molecule for analyzing stereoelectronic effects (anomeric and gauche). Here this aspect is explored.
The conformational preference of s-cis amides.
Sunday, February 10th, 2013Amides with an H-N group are a component of the peptide linkage (O=C-NH). Here I ask what the conformation (it could also be called a configuration) about the C-N bond is. A search of the following type can be defined:
The conformation of acetaldehyde: a simple molecule, a complex explanation?
Friday, February 8th, 2013Consider acetaldehyde (ethanal for progressive nomenclaturists). What conformation does it adopt, and why? This question was posed of me by a student at the end of a recent lecture of mine. Surely, an easy answer to give? Read on …
σ-π-Conjugation: seeking evidence by a survey of crystal structures.
Sunday, February 3rd, 2013The electronic interaction between a single bond and an adjacent double bond is often called σ-π-conjugation (an older term for this is hyperconjugation), and the effect is often used to e.g. explain why more highly substituted carbocations are more stable than less substituted ones. This conjugation is more subtle in neutral molecules, but following my use of crystal structures to explore the so-called gauche effect (which originates from σ-σ-conjugation), I thought I would have a go here at seeing what the crystallographic evidence actually is for the σ-π-type.