Archive for the ‘Interesting chemistry’ Category
Monday, May 31st, 2010
Something important happened in chemistry for the first time about 100 years ago. A molecule was built (nowadays we would say synthesized) specifically for the purpose of investigating a theory. It was cyclo-octatetraene or (CH)8, and it was made by Willstätter and Waser[1] to try to find out if benzene, (CH)6, was an aromatic one-off or whether it might be a member of a series, envisaged as (CH)n. Of course, a hell of a surprise was in store for Willstätter and Waser[1]! Prior to this synthesis, (CH)8 had never existed; nature had not gotten there first. In that sense, chemistry became much like mathematics had before it; it was OK to make molecules because they might be interesting, and for the purpose of investigating possible patterns in nature. So it is in this spirit that I suggest an interesting molecule here. It is a molecular trefoil, constructed by joining 15 pyrrole units together into a ring with appropriate linkers and in effect tying a knot in that ring. A trefoil knot to be specific.
(more…)
References
- R. Willstätter, and E. Waser, "Über Cyclo‐octatetraen", Berichte der deutschen chemischen Gesellschaft, vol. 44, pp. 3423-3445, 1911. https://doi.org/10.1002/cber.191104403216
Tags:Interesting chemistry, Linking number, metal cation, Trefoil knot, writhe
Posted in Interesting chemistry | 9 Comments »
Wednesday, May 26th, 2010
In the first part of the post on this topic, I described how an asymmetric sulfoxide could be prepared as a pure enantiomer using a chiral oxygen transfer reagent. In the second part, we now need to deliver a different group, cyano, to a specific face of the previously prepared sulfoxide-imine. The sulfoxide is now acting as a chiral auxilliary, and helps direct the delivery of the cyanide group to specifically one face of the imine rather than the other. After removal of the aluminum carrier for the cyano group and hydrolysis of the cyano group to a carboxylic acid group, we end up with an enantiomerically pure amino acid.
(more…)
Tags:aluminum carrier, carboxylic acid, chiroptical, cyano, free energy, Interesting chemistry
Posted in Interesting chemistry | No Comments »
Monday, May 24th, 2010
The assembly of a molecule for a purpose has developed into an art form, one arguably (chemists always argue) that is approaching its 100th birthday (DOI: 10.1002/cber.191104403216) celebrating Willstätter’s report of the synthesis of cyclo-octatetraene. Most would agree it reached its most famous achievement with Woodward’s synthesis of quinine (DOI: 10.1021/ja01221a051) in 1944. To start with, the art was in knowing how and in which order to join up all the bonds of a target. The first synthesis in which (relative) stereocontrol of those bonds was the primary objective was reported in 1951 (10.1021/ja01098a039). The art can be taken one step further. It involves control of the absolute stereochemistry, involving making one enantiomer specifically (rather than the mirror image, which of course has the same relative stereochemistry). Nowadays, a synthesis is considered flawed if the enantiomeric excess (of the desired vs the undesired isomer) of such a synthesis does not achieve at least ~98%. It is routine. But ask the people who design such syntheses if they know exactly the reasons why their reaction has succeeded, you may get a less precise answer (or just a lot of handwaving; chemists also like to wave their hands as well as argue).
(more…)
Tags:energy, free energy, Interesting chemistry, natural product, synthetic chemist
Posted in Interesting chemistry | No Comments »
Tuesday, May 4th, 2010
In this previous blog post I wrote about one way in which we have enhanced the journal article. Associated with that enhancement, and also sprinkled liberally throughout this blog, are links to a Digital Repository (if you want to read all about it, see DOI: 10.1021/ci7004737). It is a fairly specific repository for chemistry, with about 5000 entries. These are mostly the results of quantum mechanical calculations on molecules (together with a much smaller number of spectra, crystal structure and general document depositions). Today, with some help (thanks Matt!), I decided to take a look at how much use the repository was receiving.
(more…)
Tags:chemical repository, Google, Interesting chemistry, Microsoft, opendata, Skolnik, web spiders, Yahoo
Posted in Interesting chemistry | 5 Comments »
Sunday, May 2nd, 2010
Peter Murray-Rust in his blog asks for examples of the Scientific Semantic Web, a topic we have both been banging on about for ten years or more (DOI: 10.1021/ci000406v). What we are seeking of course is an example of how scientific connections have been made using inference logic from semantically rich statements to be found on the Web (ideally connections that might not have previously been spotted by humans, and lie overlooked and unloved in the scientific literature). Its a tough cookie, and I look forward to the examples that Peter identifies. Meanwhile, I thought I might share here a semantically rich molecule. OK, I identified this as such not by using the Web, but as someone who is in the process of delivering an undergraduate lecture course on the topic of conformational analysis. This course takes the form of presenting a set of rules or principles which relate to the conformations of molecules, and which themselves derive from quantum mechanics, and then illustrating them with selected annotated examples. To do this, a great many semantic connections have to be made, and in the current state of play, only a human can really hope to make most of these. We really look to the semantic web as it currently is to perhaps spot a few connections that might have been overlooked in this process. So, below is a molecule, and I have made a few semantic connections for it (but have not actually fully formalised them in this blog; that is a different topic I might return to at some time). I feel in my bones that more connections could be made, and offer the molecule here as the fuse!
(more…)
Tags:chair, chemical connections, Chemical IT, chemical world, chemist, energy, Fe, General, Interesting chemistry, lowest thermodynamic free energy, organic chemist, organometallic chemist, Peter Murray-Rust, semantic web, unusual
Posted in Chemical IT, General, Interesting chemistry | 2 Comments »
Tuesday, April 6th, 2010
Here I offer another spin-off from writing a lecture course on conformational analysis. This is the famous example of why 1,2-difluoroethane adopts a gauche rather than antiperiplanar conformation.
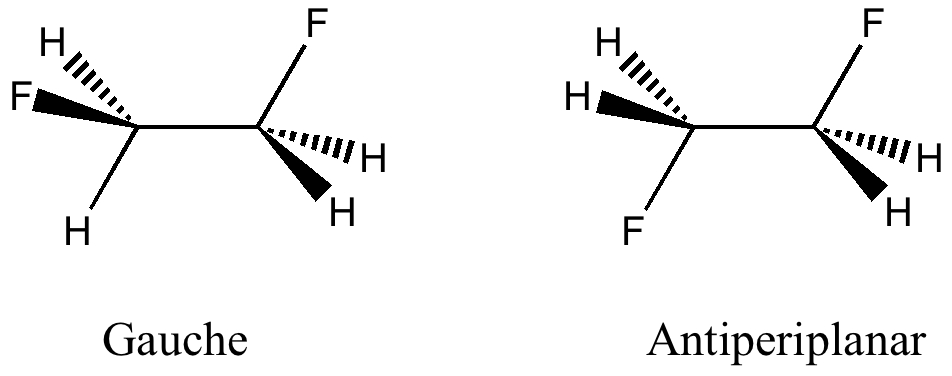
The gauche and antiperiplanar conformations of 1,2-difluoroethane
(more…)
Tags:appropriate algorithms, conformational analysis, energy gap, Here I, interaction energy, Interesting chemistry, spin-off
Posted in Interesting chemistry | 14 Comments »
Friday, April 2nd, 2010
One of the (not a few) pleasures of working in a university is the occasional opportunity that arises to give a new lecture course to students. New is not quite the correct word, since the topic I have acquired is Conformational analysis. The original course at Imperial College was delivered by Derek Barton himself about 50 years ago (for articles written by him on the topic, see DOI 10.1126/science.169.3945.539 or the original 10.1039/QR9561000044), and so I have had an opportunity to see how the topic has evolved since then, and perhaps apply some quantitative quantum mechanical interpretations unavailable to Barton himself.
(more…)
Tags:10.1021, conformational analysis, Derek Barton, energy maxima, Imperial College, Interesting chemistry, lower energy, overall energy, potential energy surface, Tutorial material
Posted in Interesting chemistry | 11 Comments »
Saturday, March 13th, 2010
One future vision for chemistry over the next 20 years or so is the concept of having machines into which one dials a molecule, and as if by magic, the required specimen is ejected some time later. This is in some ways an extrapolation of the existing peptide and nucleotide synthesizer technologies and sciences. A pretty significant extrapolation, suitable no doubt for a grand future challenge in chemistry (although the concept of tumbling a defined collection of atoms in a computer model and seeing what interesting molecules emerge, dubbed with some sense of humour as mindless chemistry, is already being done; see DOI: 10.1021/jp057107z).
(more…)
Tags:free energy, free energy barrier, Interesting chemistry, metal catalysts, nucleotide synthesizer technologies, similar energy
Posted in Interesting chemistry | No Comments »
Sunday, February 21st, 2010
Stoyanov, Stoyanova and Reed recently published on the structure of the hydrogen ion in water. Their model was H(H2O)n+, where n=6 (DOI: 10.1021/ja9101826). This suggestion was picked up by Steve Bachrach on his blog, where he added a further three structures to the proposed list, and noted of course that with this type of system there must be a fair chance that the true structure consists of a well-distributed Boltzmann population of a number of almost iso-energetic forms.
(more…)
Tags:animation, gas phase model, General, Interesting chemistry, Steve Bachrach
Posted in General, Interesting chemistry | 1 Comment »
