In the previous post, I looked at a class of molecule known as hexaphyrins, inspecting bond length alternation (BLA) at the so-called meso position, the carbon atom joining two pyrrole rings. A search of the difference in bond lengths at this position had shown two significant clusters of crystal structures.
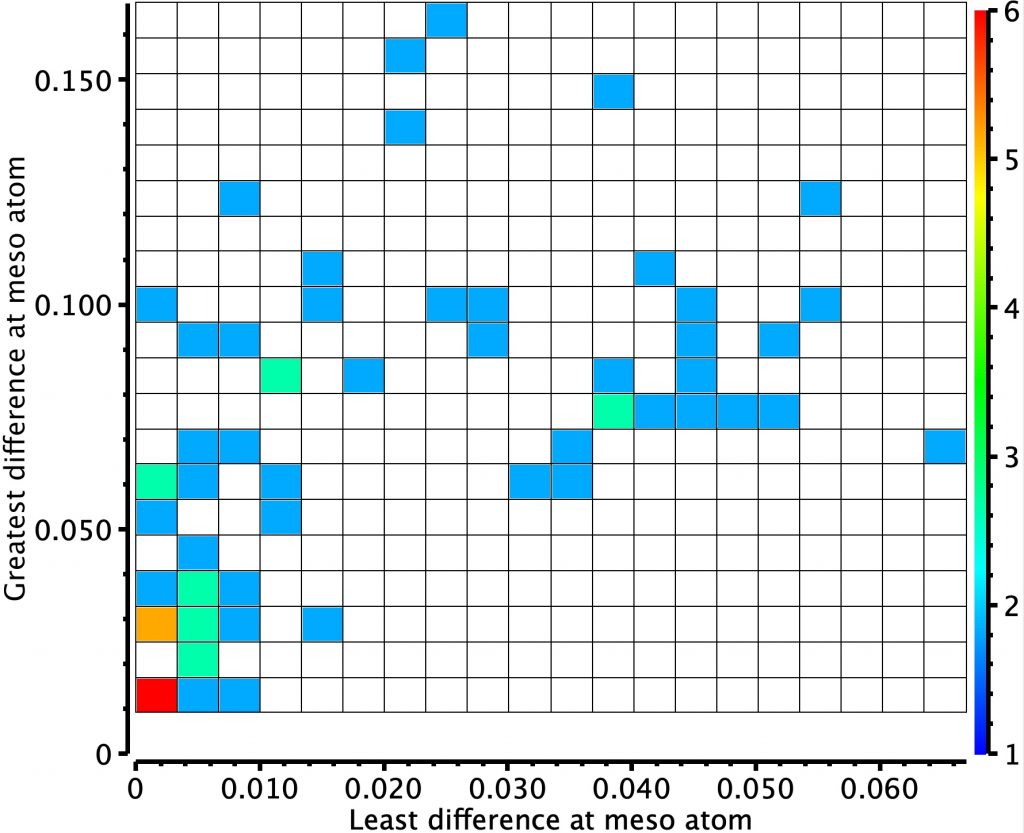 Molecules in the bottom left of this diagram shows little or no bond length alternation. The right middle shows another cluster with more extreme (and unequal) bond length alternation. I have selected one molecule from this cluster, EGIJEK and it differs from EGIHUY in having four NH units in the ring, whereas the latter has only two.
Molecules in the bottom left of this diagram shows little or no bond length alternation. The right middle shows another cluster with more extreme (and unequal) bond length alternation. I have selected one molecule from this cluster, EGIJEK and it differs from EGIHUY in having four NH units in the ring, whereas the latter has only two.
Archive for the ‘Interesting chemistry’ Category
Bond length alternation (BLA) in large conjugated rings: an (anti-aromatic) update.
Thursday, October 3rd, 2019Bond length alternation (BLA) in large aromatic rings: an experimental reality check.
Monday, September 30th, 2019The theme of the last three posts derives from the recently reported claimed experimental observation of bond length alternation (BLA) in cyclo[18]carbon, a ring of just 18 carbon atoms.[cite]10.1126/science.aay1914[/cite] Having found that different forms of quantum calculation seem to find this property particularly difficult to agree upon, not only for cyclocarbon but for twisted lemniscular annulenes (which contain CH rather than just C units), I thought it might be time to look at some more experimental data and my chosen system is a class called the hexaphyrins, of which there are a number of experimental crystal structures.
The Kekulé vibration as a function of aromatic ring size. A different perspective using lemniscular rings.
Friday, September 27th, 2019In the previous posts, I tried to track down the onset of bond length alternation (BLA) as a function of ring size in aromatic cyclocarbons, finding the answer varied dramatically depending on the type of method used to calculate it. So here I change the system to an unusual kind of aromatic ring, the leminiscular or figure-eight annulene series.♥ I explore the Kekulé vibration for such species for which a 4n+2 π electron count means they are cyclically Möbius aromatic.[cite]10.1016/j.comptc.2014.09.028[/cite]
Cyclo[6] and [10]carbon. The Kekulé vibrations compared.
Tuesday, September 3rd, 2019In the previous post, I looked at the so-called Kekulé vibration of cyclo[18]carbon using various quantum methods and basis sets. Because some of these procedures can take a very long time, I could not compare them using the same high-quality consistent atom basis set for the carbon (Def2-TZVPP). Here I try to start to do this using the smaller six and ten carbon rings to see what trends might emerge. FAIR data are at DOI: 10.14469/hpc/6069
Cyclo[18]carbon: The Kekulé vibration calculated and hence a mystery!
Friday, August 30th, 2019I have discussed the vibration in benzene known as the Kekulé mode in other posts, the first of which was all of ten years ago. It is a stretching mode that lengthens three of the bonds in benzene (a [6]-annulene) and shortens the other three, thus leading to a cyclohexatriene motif (see below). This vibration is real (+ve force constant) in benzene itself, which indicates that distorting the structure from six to three-fold symmetry leads to an increase in energy. Benzene therefore has a symmetrising influence, and it comes as a surprise to most to learn that this is actually due to the σ rather than the π-electrons! But there are good reasons to believe that as the ring size of the annulene increases, the Kekulé vibration will evolve from a real mode into an imaginary (-ve force constant) vibration representing a transition state for mutating the single and double bonds. At some point therefore, the more symmetrical geometry of the annulene in which all the bonds are of equal length will change into one of lower symmetry, in which BLA (bond length alternation) occurs and the symmetrical form becomes a transition state for this process.
A Non-nitrogen Containing Morpholine Isostere; an application of FAIR data principles.
Sunday, August 4th, 2019In the pipeline reports on an intriguing new ring system acting as an isostere for morpholine. I was interested in how the conformation of this ring system might be rationalised electronically and so I delved into the article.[cite]10.1021/acs.jmedchem.9b00348[/cite] Here I recount what I found.
Anniversaries: The World-Wide-Web at 30 and 25 (+ CERN’s LHC as a bonus).
Saturday, June 15th, 2019
The World-Wide-Web is currently celebrating its 30th anniversary; you can get the T-shirt in the CERN visitor centre! Five years on, in May 1994, the first Web conference took place (WWW94) at CERN and now celebrating its own 25th anniversary. That 1994 conference also had various break-out sessions, one of which summarised the state of chemistry on the web at the time. You can see my general but entirely personal impressions written after the workshop (DOI: 10.14469/hpc/5850), with a chemistry specific version at DOI: 10.14469/hpc/5851. A real trip down memory lane and an indication of how much has happened in 25 years!
ChemRxiv. Why?
Wednesday, June 5th, 2019In August 2016, the launch of a chemistry pre-print service ChemRxiv was announced. I was phoned a day or so later by a staff journalist at C&E News for my opinion. The only comment that was retained for their report was my instantaneous feeling that “the community needed a chemistry pre-print server like one needed a hole in the head“. I had been there before you see, recollecting a pre-print server launched by the ChemWeb service around 1996 or 1997 and which lasted only about two years before being withdrawn due to the low quality of the preprints. So what do I think of ChemRxiv now in 2019?
Diatomics with eight valence-electrons: formation by radioactive decay.
Sunday, June 2nd, 2019This is a follow up to my earlier post about C⩸N+, itself inspired by this ChemRxiv pre-print[cite]10.26434/chemrxiv.8009633.v1[/cite] which describes a chemical synthesis of singlet biradicaloid C2 and its proposed identification as such by chemical trapping.
Startling bonds: revisiting C⩸N+, via the helium bond in N≡C-He+.
Monday, May 27th, 2019Although the small diatomic molecule known as dicarbon or C2 has been known for a long time, its properties and reactivity have really only been determined via its very high temperature generation. My interest started in 2010, when I speculatively proposed here that the related isoelectronic species C⩸N+ might sustain a quadruple bond. Shortly thereafter, a torrent of theoretical articles started to appear in which the idea of a quadruple bond to carbon was either supported or rejected. Clearly more experimental evidence was needed. The recent appearance of a Chemrxiv pre-print entitled “Room-temperature chemical synthesis of C2“.[cite]10.26434/chemrxiv.8009633.v1[/cite] claims to provide just this! Using the synthetic scheme outlined below, they trapped “C2” with a variety of reagents (see Figure 2A in their article), concluding that the observed reactivity best matched that of singlet “biradicaloid” C2 sustaining a quadruple bond.