In 1890, chemists had to work hard to find out what the structures of their molecules were, given they had no access to the plethora of modern techniques we are used to in 2010. For example, how could they be sure what the structure of naphthalene was? Well, two such chemists, William Henry Armstrong (1847-1937) and his student William Palmer Wynne (1861-1950; I might note that despite working with toxic chemicals for years, both made it to the ripe old age of ~90!) set out on an epic 11-year journey to synthesize all possible mono, di, tri and tetra-substituted naphthalenes. Tabulating how many isomers they could make (we will call them AW here) would establish beyond doubt the basic connectivity of the naphthalene ring system. This was in fact very important, since many industrial dyes were based on this ring system, and patents depended on getting it correct! Amazingly, their collection of naphthalenes survives to this day. With the passage of 120 years, we can go back and check their assignments. The catalogued collection (located at Imperial College) comprises 263 specimens. Here the focus is on just one, specimen number number 22, which bears an original label of trichloronaphthalene [2:3:1] and for which was claimed a melting point of 109.5°C. What caught our attention is that a search for this compound in modern databases (Reaxys if you are interested, what used to be called Beilstein) reveals the compound to have a melting point of ~84°C. So, are alarm bells ringing? Did AW make a big error? Were many of the patented dyes not what they seemed?
Archive for the ‘Interesting chemistry’ Category
A historical detective story: 120 year old crystals
Wednesday, November 17th, 2010The strongest bond in the universe!
Sunday, October 24th, 2010The rather presumptious title assumes the laws and fundamental constants of physics are the same everywhere (they may not be). With this constraint (and without yet defining what is meant by strongest), consider the three molecules: (more…)
Secrets of a university admissions interviewer
Sunday, September 19th, 2010Many university chemistry departments, and mine is no exception, like to invite applicants to our courses to show them around. Part of the activities on the day is an “interview” in which the candidate is given a chance to shine. Over the years, I have evolved questions about chemistry which can form the basis of discussion, and I thought I would share one such question here. It starts by my drawing on the blackboard (yes, I really still use one!) the following molecular structure.
Solid carbon dioxide: hexacoordinate carbon?
Friday, September 17th, 2010Carbon dioxide is much in the news, not least because its atmospheric concentration is on the increase. How to sequester it and save the planet is a hot topic. Here I ponder its solid state structure, as a hint to its possible reactivity, and hence perhaps for clues as to how it might be captured. The structure was determined (DOI 10.1103/PhysRevB.65.104103) as shown below.
The oldest reaction mechanism: updated!
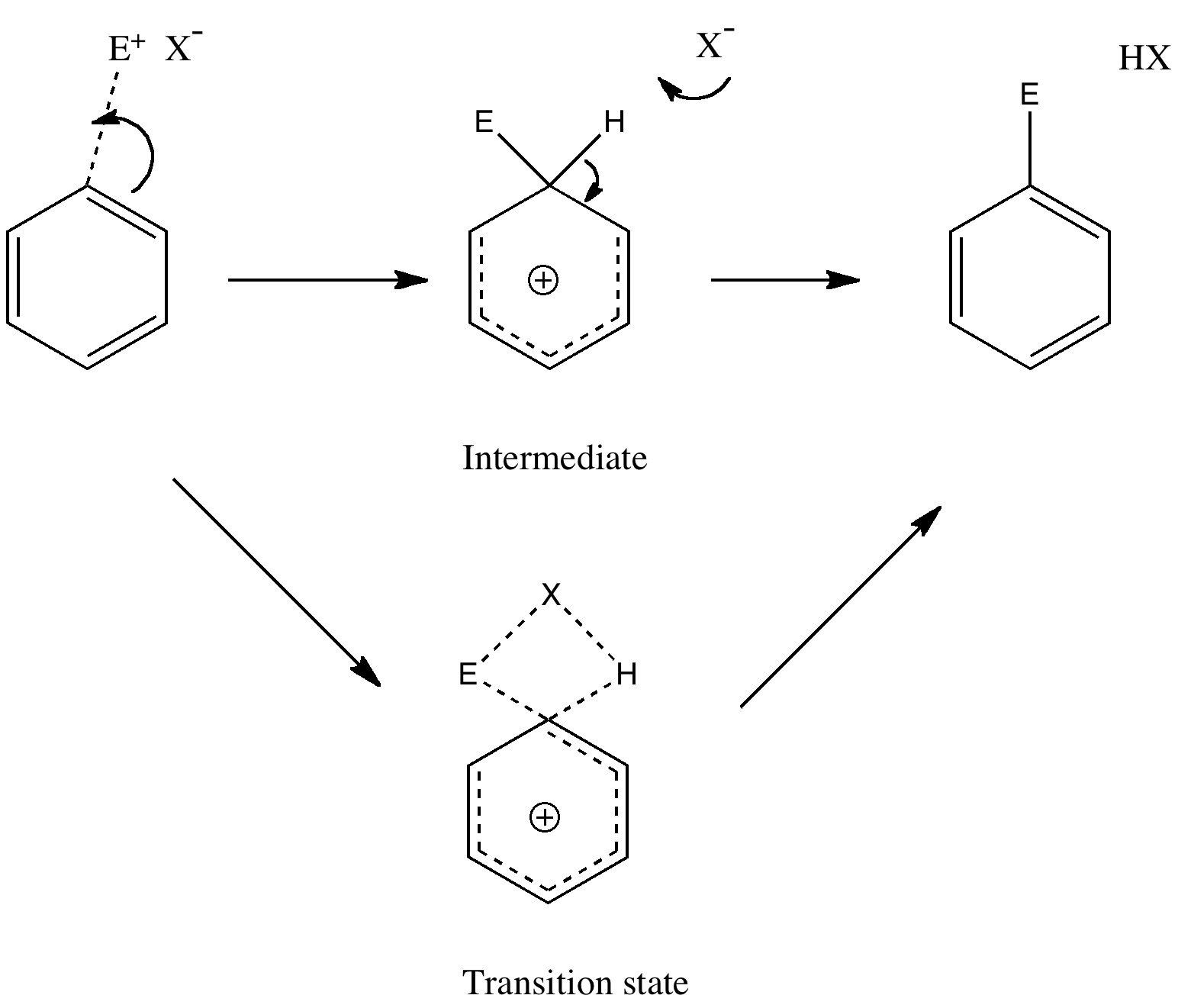
Tuesday, September 14th, 2010Unravelling reaction mechanisms is thought to be a 20th century phenomenon, coincident more or less with the development of electronic theories of chemistry. Hence electronic arrow pushing as a term. But here I argue that the true origin of this immensely powerful technique in chemistry goes back to the 19th century. In 1890, Henry Armstrong proposed what amounts to close to the modern mechanism for the process we now know as aromatic electrophilic substitution [cite]10.1039/PL8900600095[/cite]. Beyond doubt, he invented what is now known as the Wheland Intermediate (about 50 years before Wheland wrote about it, and hence I argue here it should really be called the Armstrong/Wheland intermediate). This is illustrated (in modern style) along the top row of the diagram.
Bio-renewable green polymers: Stereoinduction in poly(lactic acid)
Saturday, July 24th, 2010Lactide is a small molecule made from lactic acid, which is itself available in large quantities by harvesting plants rather than drilling for oil. Lactide can be turned into polymers with remarkable properties, which in turn degrade down easily back to lactic acid. A perfect bio-renewable material!
The weirdest bond of all? Laplacian isosurfaces for [1.1.1]Propellane.
Wednesday, July 21st, 2010In this post, I will take a look at what must be the most extraordinary small molecule ever made (especially given that it is merely a hydrocarbon). Its peculiarity is the region indicated by the dashed line below. Is it a bond? If so, what kind, given that it would exist sandwiched between two inverted carbon atoms?