If H3N+-O– is viable compared with its tautomer H2N-OH when carrying water bridges, then why not try H2O+-O– vs HO-OH?
Archive for the ‘Interesting chemistry’ Category
Oxane oxide: a tautomer of hydrogen peroxide.
Friday, April 15th, 2016Azane oxide, a tautomer of hydroxylamine.
Friday, April 15th, 2016In the previous post I described how hydronium hydroxide or H3O+…HO–, an intermolecular tautomer of water, has recently been observed captured inside an organic cage[cite]10.1002/chem.201406383[/cite] and how the free-standing species in water can be captured computationally with the help of solvating water bridges. Here I explore azane oxide or H3N+-O–,‡ a tautomer of the better known hydroxylamine (H2N-OH).
Hydronium hydroxide: the why of pH 7.
Thursday, April 14th, 2016Ammonium hydroxide (NH4+…OH–) can be characterised quantum mechanically when stabilised by water bridges connecting the ion-pairs. It is a small step from there to hydronium hydroxide, or H3O+…OH–. The measured concentrations [H3O+] ≡ [OH–] give rise of course to the well-known pH 7 of pure water, and converting this ionization constant to a free energy indicates that the solvated ion-pair must be some ~19.1 kcal/mol higher in free energy than water itself.♣ So can a quantum calculation reproduce pH7 for water?
Earth’s missing chemistry.
Wednesday, February 24th, 2016At the precise moment I write this, there is information about 108,230,950 organic and inorganic chemical substances from the World's disclosed chemistry. So it was with a sense of curiosity that I came across this article in the American Mineralogist[cite]/10.2138/am-2015-5417[/cite] entitled "Earth’s “missing” minerals" (the first in a series of articles apparently planned on the topic of the missing ones). The abstract is particularly interesting and whilst I encourage you to go read the article itself, I will quote some eye-catching observations from just this abstract:
I’ve started so I’ll finish. Kinetic isotope effect models for a general acid as a catalyst in the protiodecarboxylation of indoles.
Sunday, January 10th, 2016Earlier I explored models for the heteroaromatic electrophilic protiodecarboxylation of an 3-substituted indole, focusing on the role of water as the proton transfer and delivery agent. Next, came models for both water and the general base catalysed ionization of indolinones. Here I explore general acid catalysis by evaluating the properties of two possible models for decarboxylation of 3-indole carboxylic acid, one involving proton transfer (PT) from neutral water in the presence of covalent un-ionized HCl (1) and one with PT from a protonated water resulting from ionised HCl (2).
I’ve started so I’ll finish. The mechanism of diazo coupling to indoles – forty (three) years on!
Thursday, December 24th, 2015
The BBC TV quiz series Mastermind was first broadcast in the UK in 1972, the same time I was starting to investigate the mechanism of diazocoupling to substituted indoles as part of my Ph.D. researches. The BBC program became known for the catch phrase I've started so I'll finish; here I will try to follow this precept with the project I started then. In 1972, one measured the rates of chemical reactions to gain insights into the transition state kinetic model. To obtain more data, we used isotopes such as 2H or 3H, together with substituents such as R-t-butyl to modify the potential energy surfaces of the reactions by inducing steric effects.[cite]10.1039/P29750001209[/cite],[cite]10.5281/zenodo.18777[/cite] We found that the kinetics for this reaction were actually complex‡ (in part because of pH dependence) involving a Wheland intermediate (the formation of which is shown with red curly arrows above) followed by the collapse of this intermediate to the diazo-coupled product (blue arrows). Coupling to 2-methyl indole (R=X=H, R'=Me), 2-t-butyl indole (R=H, R'=t-butyl) and 4-methyl-2-t-butyl indole (R=Me, R'=t-butyl) revealed that the kinetic isotope effects induced by replacing H by D or T were "not apparent" (i.e. close to 1), the inference being that the rate constant k1 for those systems was slower than k2; the formation of the Wheland intermediate was rate determining (the rds) for the reaction. But with 2-methyl-4,6-di-t-butyl indole (R=t-butyl, R'=Me) this changed and a deuterium isotope effect of ~7 was observed. The rate determining proton removal from the Wheland intermediate k2 was now slower than k1. With 2,4,6-tri-t-butyl indole, we ended by noting that the reaction become almost too slow to observe and furthermore was accompanied by loss of a t-butyl cation as well as a proton. At this point we attempted to infer some transition state models consistent with these observations. Note that we had relatively little data with which to derive our 3D models (one needs to define a geometry using 3N-6 variables, along with its relative energy and force constants). The text and diagram of our attempt is shown below.
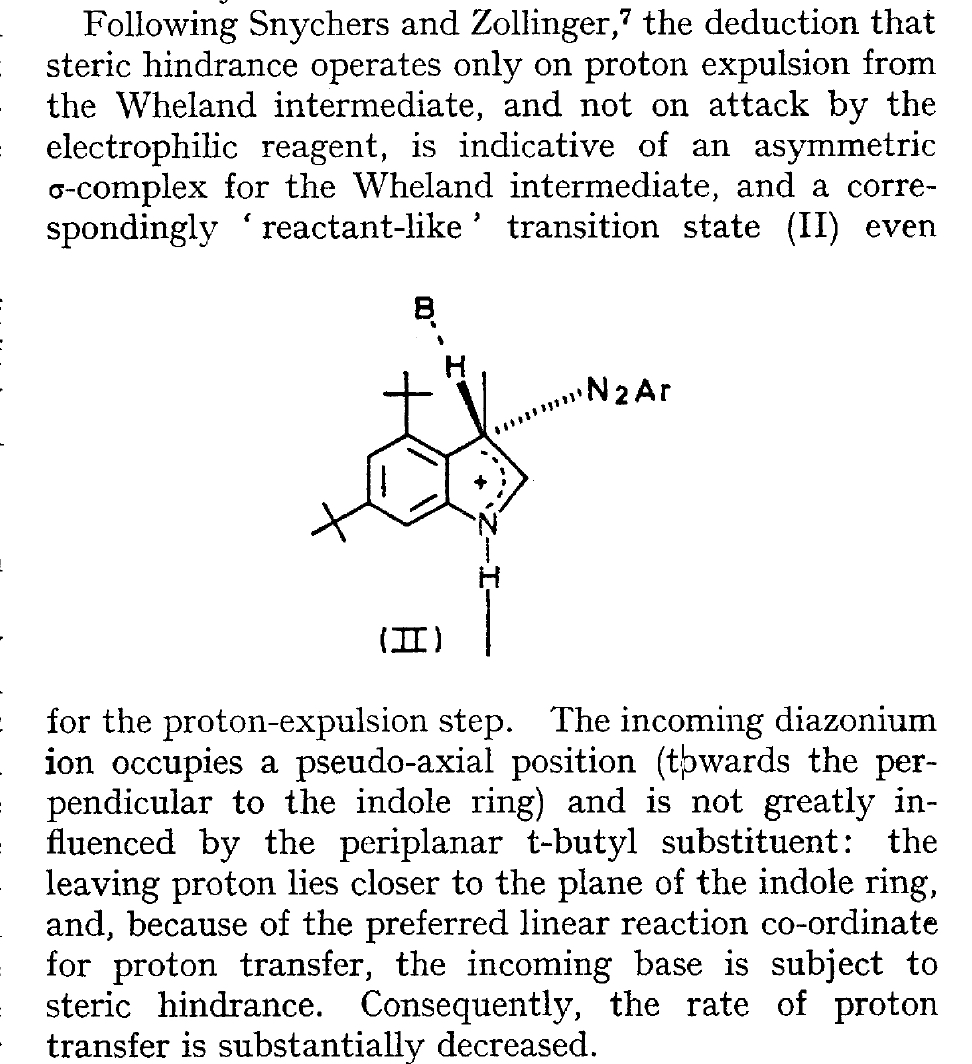 The main points of this argument were;
The main points of this argument were;