Each year, C&E News runs a poll for their “Molecule of the year“. I occasionally comment with some aspect of one of the molecules that catches my eye (I have already written about cyclo[18]carbon, another in the list). Here, it is the Incredible chloride cage, a cryptand-like container with an attomolar (1017 M-1) affinity for a chloride anion.[cite]10.1126/science.aaw5145[/cite] The essence of the binding is six short CH…Cl– and one slightly longer interactions to the same chloride (DOI: 10.5517/ccdc.csd.cc1ngqrl) and one further hydrogen bond to a water molecule; eight coordinated chloride anion!
Archive for the ‘Interesting chemistry’ Category
Molecules of the year – 2019: the incredible chloride cage.
Friday, December 13th, 2019Sign inversions in optical rotation as a function of wavelength (ORD spectra)
Monday, December 9th, 2019I have been discussing some historical aspects of the absolute configuration of molecules and how it was connected to their optical rotations. The nomenclature for certain types of molecules such as sugars and less commonly amino acids includes the notation (+) to indicate that the specific optical rotation of the molecule has a positive (rather than a negative) value. What is rarely mentioned is the implicit wavelength at which the rotation is measured. Historically polarimeters operated at the so-called sodium Fraunhofer D-line (588.995nm, hence the name [α]D) and only much more recently at the mercury e-line (546.073nm). The former was used for uncoloured organic molecules, since it was realised early on that colour and optical rotation did not mix well. Here I take a closer look at this aspect by constructing the hypothetical molecule shown below.
What effect do explicit solvent molecules have on calculated optical rotation: D-(“+”)-Glyceraldehyde.
Saturday, December 7th, 2019In this series of posts on optical rotations, I firstly noted Kirkwood’s 1937 attempt to correlate the optical rotation of butan-2-ol with its absolute configuration. He had identified as essential knowing the relative orientation (the term conformation was not yet in common use) of the two methyl groups (the modern terms are gauche vs anti) and also that of the hydroxyl group, noting that anisotropy from this group could influence his result (he had assumed it was linear, or axially symmetric). I then looked at D-(+)-glyceraldehyde, noting that this species itself has a strongly negative rotation and that it is the hydrated diol that results in a positive rotation and hence the (+) designation. Here I take another look at this latter system to see what effect adding explicit water molecules to the unhydrated form of glyceraldehyde might have on its computed rotation, on the premise that strong hydrogen bonds can also contribute anisotropy to the system.
The Structure of Tetrodotoxin as a free base – with a better solvation model.
Tuesday, November 26th, 2019In the previous post, I discussed the structure of the free base form of tetrodotoxin, often represented as originally suggested by Woodward[cite]10.1351/pac196409010049[/cite] below in an ionic form:
The Structure of Tetrodotoxin as a free base.
Saturday, November 9th, 2019The notorious neurotoxin Tetrodotoxin is often chemically represented as a zwitterion, shown below as 1. This idea seems to originate from a famous article written in 1964 by the legendary organic chemist, Robert Burns Woodward.[cite]10.1351/pac196409010049[/cite] This structure has propagated on to Wikipedia and is found in many other sources.
With the elegance and the unique style that is typical Woodward, his article is a tour de force because of the way in which he deploys a large armoury of spectroscopic (X-ray crystal,† NMR, IR) as well as physicochemical (pKa) tools to infer this structure; an approach that has been subsequently widely emulated. The article a well worth a read for the elegant logic that slowly builds to a climax on page 73 (sic!) of the article, when he unveils his final structure (XXXVIII, or 38). The lecture(s) from which the article is apparently derived must have been one hell of an occasion.‡
Does Kekulene have Kekulé vibrational modes? Yes!
Saturday, October 19th, 2019Increasingly, individual small molecules are having their structures imaged using STM, including cyclo[18]carbon that I recently discussed. The latest one receiving such treatment is Kekulene.[cite]10.1021/jacs.9b07926[/cite]
Bond length alternation (BLA) in large conjugated rings: an (anti-aromatic) update.
Thursday, October 3rd, 2019In the previous post, I looked at a class of molecule known as hexaphyrins, inspecting bond length alternation (BLA) at the so-called meso position, the carbon atom joining two pyrrole rings. A search of the difference in bond lengths at this position had shown two significant clusters of crystal structures.
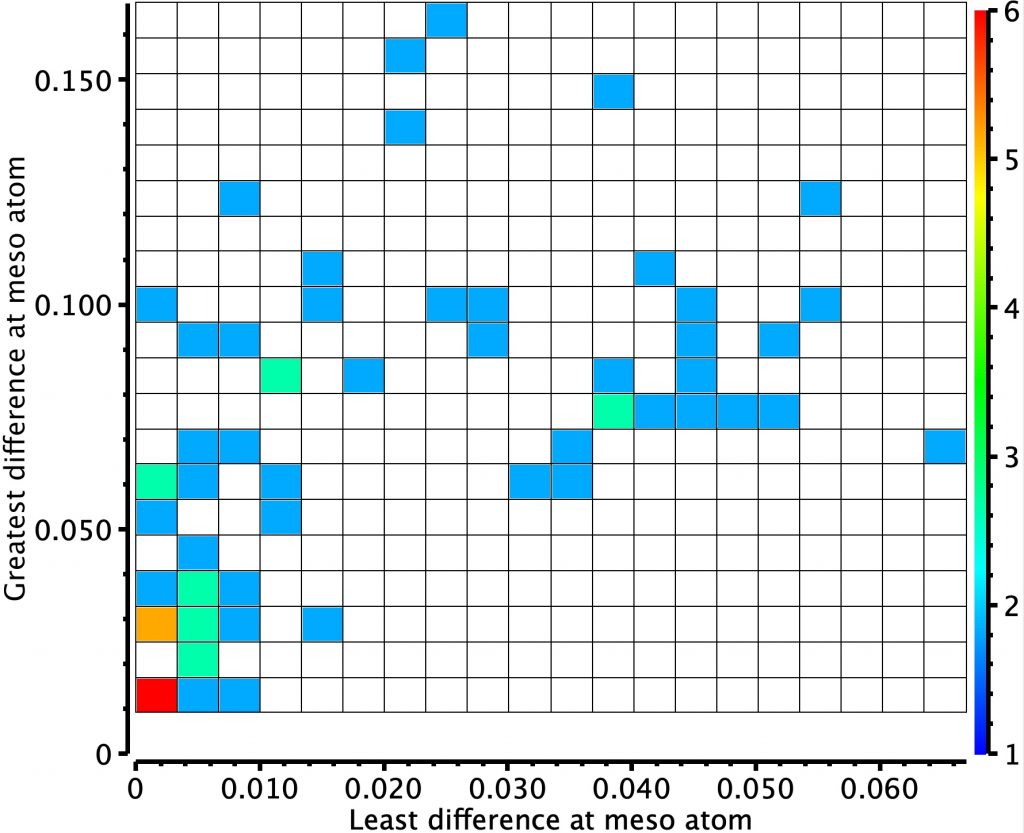 Molecules in the bottom left of this diagram shows little or no bond length alternation. The right middle shows another cluster with more extreme (and unequal) bond length alternation. I have selected one molecule from this cluster, EGIJEK and it differs from EGIHUY in having four NH units in the ring, whereas the latter has only two.
Molecules in the bottom left of this diagram shows little or no bond length alternation. The right middle shows another cluster with more extreme (and unequal) bond length alternation. I have selected one molecule from this cluster, EGIJEK and it differs from EGIHUY in having four NH units in the ring, whereas the latter has only two.
Bond length alternation (BLA) in large aromatic rings: an experimental reality check.
Monday, September 30th, 2019The theme of the last three posts derives from the recently reported claimed experimental observation of bond length alternation (BLA) in cyclo[18]carbon, a ring of just 18 carbon atoms.[cite]10.1126/science.aay1914[/cite] Having found that different forms of quantum calculation seem to find this property particularly difficult to agree upon, not only for cyclocarbon but for twisted lemniscular annulenes (which contain CH rather than just C units), I thought it might be time to look at some more experimental data and my chosen system is a class called the hexaphyrins, of which there are a number of experimental crystal structures.
The Kekulé vibration as a function of aromatic ring size. A different perspective using lemniscular rings.
Friday, September 27th, 2019In the previous posts, I tried to track down the onset of bond length alternation (BLA) as a function of ring size in aromatic cyclocarbons, finding the answer varied dramatically depending on the type of method used to calculate it. So here I change the system to an unusual kind of aromatic ring, the leminiscular or figure-eight annulene series.♥ I explore the Kekulé vibration for such species for which a 4n+2 π electron count means they are cyclically Möbius aromatic.[cite]10.1016/j.comptc.2014.09.028[/cite]
Cyclo[6] and [10]carbon. The Kekulé vibrations compared.
Tuesday, September 3rd, 2019In the previous post, I looked at the so-called Kekulé vibration of cyclo[18]carbon using various quantum methods and basis sets. Because some of these procedures can take a very long time, I could not compare them using the same high-quality consistent atom basis set for the carbon (Def2-TZVPP). Here I try to start to do this using the smaller six and ten carbon rings to see what trends might emerge. FAIR data are at DOI: 10.14469/hpc/6069