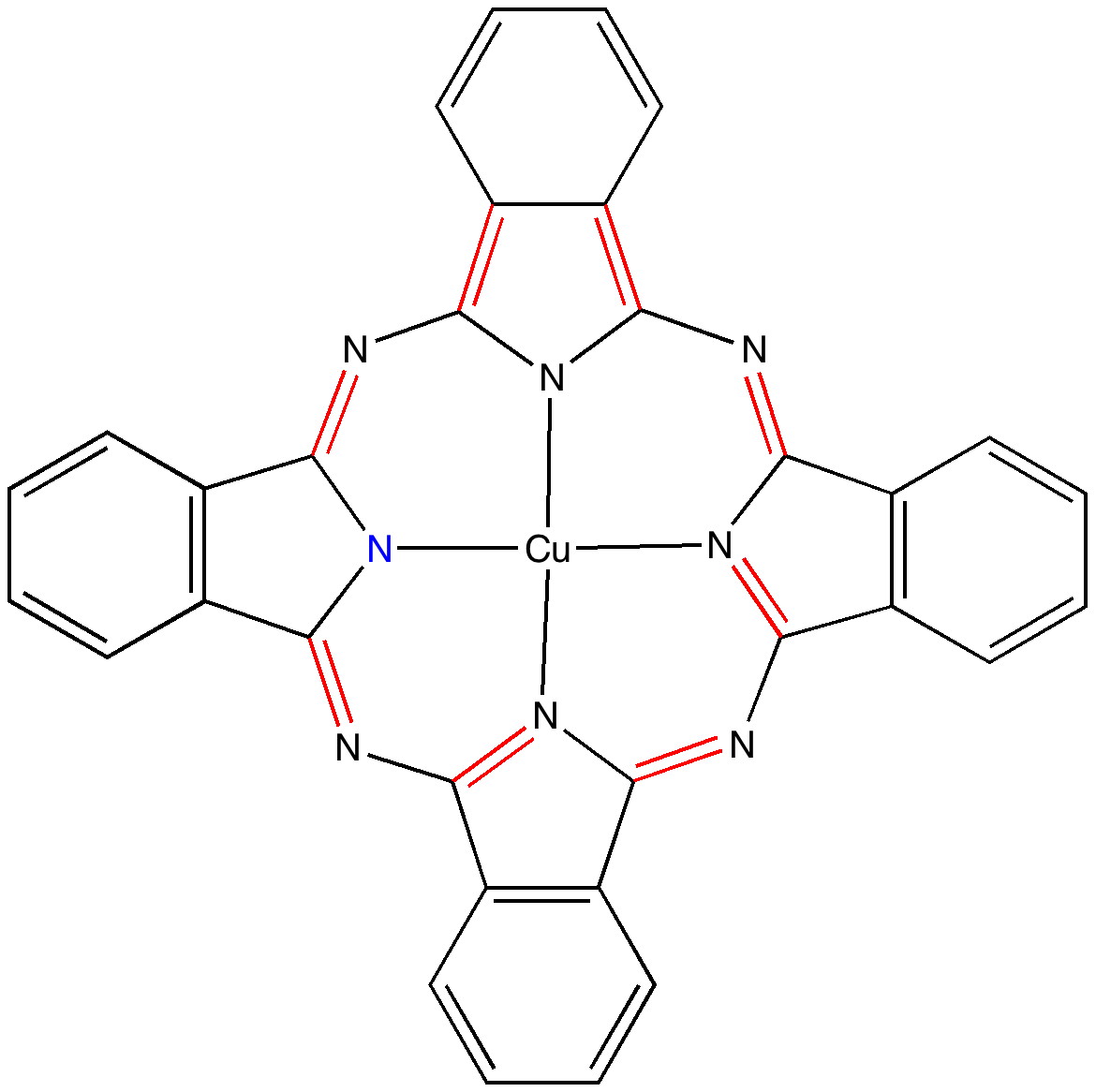
Andy Mclean posted a comment to my story of copper phthalocyanine (Monastral blue). The issue was its colour, and more specifically why this pigment has two peaks λmax 610 and 710nm making it blue. The first was accurately reproduced by calculation on the monomer, but the second was absent with such a model. Andy suggested this latter was due to stacking. Here, the calculated spectrum of a stacked dimer is explored.
Archive for the ‘Interesting chemistry’ Category
The colour of Monastral blue (part 2).
Monday, April 4th, 2011From the colour blue to molecular wires
Wednesday, March 9th, 2011In the previous post I pondered the colour of Monastral blue (copper phthalocyanine). Something did not quite fit, and so I speculated that perhaps some oxidation of the pigment might give a new species. This species (Cambridge code FEGJOQ) comprises two parts of copper phthalocyanine, 1 part of the corresponding cation, and 1 part of triodide anion. Looking at the packing of this system, I spotted something I had seen some time ago in NaI2.Acetone, namely an infinitely long and absolutely straight chain of iodine atoms, a molecular wire if you like.
Monastral: the colour of blue
Tuesday, March 8th, 2011The story of Monastral is not about a character in the Magic flute, but is a classic of chemical serendipity, collaboration between industry and university, theoretical influence, and of much else. Fortunately, much of that story is actually recorded on film (itself a unique archive dating from 1933 and being one of the very first colour films in existence!). Patrick Linstead, a young chemist then (he eventually rose to become rector of Imperial College) tells the story himself here. It is well worth watching, if only for its innocent social commentary on the English class system (and an attitude to laboratory safety that should not be copied nowadays). Here I will comment only on its colour and its aromaticity.
The thermodynamic energies of left and right handed DNA.
Saturday, March 5th, 2011In this earlier post, I noted some aspects of the calculated structures of both Z- and B-DNA duplexes. These calculations involved optimising the positions of around 250-254 atoms, for d(CGCG)2 and d(ATAT)2, an undertaking which has taken about two months of computer time! The geometries are finally optimised to the point where 2nd derivatives can be calculated, and which reveal up to 756 all-positive force constants and 6 translations and rotations which are close to zero! This now lets one compute the thermodynamic relative energies using ωB97XD/6-31G(d) (for 2nd derivatives) and 6-31G(d,p) (for dispersion terms). All geometries are optimized using a continuum solvent field (water), and are calculated, without a counterion, as hexa-anions. (more…)
Lapis lazuli: the colour of ultramarine.
Saturday, March 5th, 2011The colour of purple
Thursday, February 24th, 2011One of my chemical heroes is William Perkin, who in 1856 famously (and accidentally) made the dye mauveine as an 18 year old whilst a student of August von Hofmann, the founder of the Royal College of Chemistry (at what is now Imperial College London). Perkin went on to found the British synthetic dyestuffs and perfumeries industries. The photo below shows Charles Rees, who was for many years the Hofmann professor of organic chemistry at the very same institute as Perkin and Hofmann himself, wearing his mauveine tie. A colleague, who is about to give a talk on mauveine, asked if I knew why it was, well so very mauve. It is a tad bright for today’s tastes!
Shorter is higher: the strange case of diberyllium.
Friday, January 21st, 2011Much of chemistry is about bonds, but sometimes it can also be about anti-bonds. It is also true that the simplest of molecules can have quite subtle properties. Thus most undergraduate courses in chemistry deal with how to describe the bonding in the diatomics of the first row of the periodic table. Often, only the series C2 to F2 is covered, so as to take into account the paramagnetism of dioxygen, and the triple bonded nature of dinitrogen (but never mentioning the strongest bond in the universe!). Rarely is diberyllium mentioned, and yet by its strangeness, it can also teach us a lot of chemistry.
A comparison of left and right handed DNA double-helix models.
Saturday, January 1st, 2011When Watson and Crick (WC) constructed their famous 3D model for DNA, they had to decide whether to make the double helix left or right handed. They chose a right-handed turn, on the grounds that their attempts at left-handed models all “violated permissible van der Waals contacts“. No details of what these might have been were given in their original full article (or the particular base-pairs which led to the observation). This follow-up to my earlier post explores this aspect, using a computer model.