Archive for the ‘Interesting chemistry’ Category
Wednesday, July 25th, 2018
Consider the four reactions. The first two are taught in introductory organic chemistry as (a) a proton transfer, often abbreviated PT, from X to B (a base) and (b) a hydride transfer from X to A (an acid). The third example is taught as a hydrogen atom transfer or HAT from X to (in this example) O. Recently an article has appeared[1] citing an example of a fourth fundamental type (d), which is given the acronym cPCET which I will expand later. Here I explore this last type a bit further, in the context that X-H bond activations are currently a very active area of research.

(more…)
References
- J.E.M.N. Klein, and G. Knizia, "cPCET versus HAT: A Direct Theoretical Method for Distinguishing X–H Bond‐Activation Mechanisms", Angewandte Chemie International Edition, vol. 57, pp. 11913-11917, 2018. https://doi.org/10.1002/anie.201805511
Tags:chemical reactions, Chemistry, Deprotonation, Hydride, Hydrogen, Hydrogen atom abstraction, Proton, proton travel, Proton-coupled electron transfer, Technology/Internet
Posted in Interesting chemistry | No Comments »
Wednesday, July 18th, 2018
FAIR is one of those acronyms that spreads rapidly, acquires a life of its own and can mean many things to different groups. A two-day event has just been held in Amsterdam to bring some of those groups from the chemical sciences together to better understand FAIR. Here I note a few items that caught my attention.
(more…)
Tags:Acronym, Amsterdam, chemical sciences, City: Amsterdam, Queen Mary University of London, spectroscopy, Technology/Internet, text editor, University of London, visualisation tools
Posted in Interesting chemistry | 3 Comments »
Thursday, July 12th, 2018
This last month, as a follow-up to the preceding post on the colour of flowers, I have been moonlighting by blogging elsewhere. Do go visit my “guerrilla blog” at perivalepark.london. Part of this project involves visiting two “physic or botanic” gardens, which originate from early 17th century explorations of herbs and other botanicals as medicines. Both are very old and their chemistry is indeed fascinating; more of which later.
(more…)
Tags:Botany, Herb
Posted in Interesting chemistry | No Comments »
Monday, June 18th, 2018
It was about a year ago that I came across a profusion of colour in my local Park. Although colour in fact was the topic that sparked my interest in chemistry many years ago (the fantastic reds produced by diazocoupling reactions), I had never really tracked down the origin of colours in many flowers. It is of course a vast field. Here I take a look at just one class of molecule responsible for many flower colours, anthocyanidin, this being the sugar-free counterpart of the anthocyanins found in nature.

(more…)
Tags:Anthocyanidin, Anthocyanin, Chemistry, Delphinidin, HOMO/LUMO, Major, Molecular electronic transition, Molecule, Nature, PH indicators, Quantum chemistry, spectroscopy, Ultraviolet–visible spectroscopy
Posted in Interesting chemistry | 4 Comments »
Wednesday, May 16th, 2018
Ten years are a long time when it comes to (recent) technologies. The first post on this blog was on the topic of how to present chemistry with three intact dimensions. I had in mind molecular models, molecular isosurfaces and molecular vibrations (arguably a further dimension). Here I reflect on how ten years of progress in technology has required changes and the challenge of how any necessary changes might be kept “under the hood” of this blog.
(more…)
Tags:Ajax, Computer programming, computing, Cross-platform software, HTML, Java, Java applet, Java technology, JavaScript, JavaScript libraries, jmol, JQuery, NPAPI, Scientific Journal, Software engineering, Technology/Internet, web browser behaviour, web browsers, Web-page security
Posted in Interesting chemistry | 6 Comments »
Sunday, May 6th, 2018
The site fairsharing.org is a repository of information about FAIR (Findable, Accessible, Interoperable and Reusable) objects such as research data.
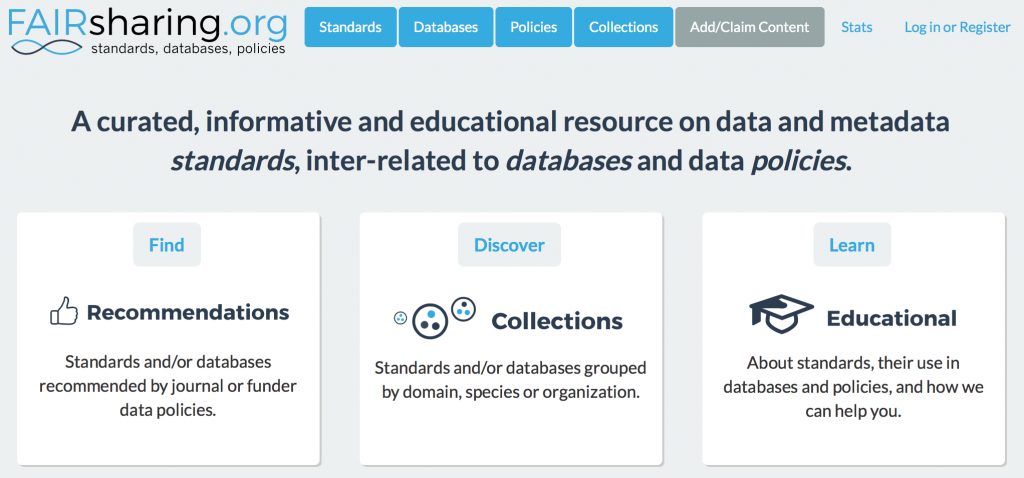
(more…)
Tags:above site, chemical components, Findability, Human behavior, Information, Information architecture, Information science, Institutional repository, journal data editor, Knowledge, Knowledge representation, Open access, Open access in Australia, Oscar, PDF, recognition software, Technology/Internet, Web design
Posted in Interesting chemistry | 2 Comments »
Wednesday, April 18th, 2018
The molecules below were discussed in the previous post as examples of highly polar but formally neutral molecules, a property induced by aromatisation of up to three rings. Since e.g. compound 3 is known only in its protonated phenolic form, here I take a look at the basicity of the oxygen in these systems to see if deprotonation of the ionic phenol form to the neutral polar form is viable.
(more…)
Tags:Antiseptics, Aromatization, Chemistry, energy, energy minimum, Hydrogen, Molecule, Neurotoxins, Science
Posted in Interesting chemistry | No Comments »
Friday, April 13th, 2018
In several posts a year or so ago I considered various suggestions for the most polar neutral molecules, as measured by the dipole moment. A record had been claimed[1] for a synthesized molecule of ~14.1±0.7D. I pushed this to a calculated 21.7D for an admittedly hypothetical and unsynthesized molecule. Here I propose a new family of compounds which have the potential to extend the dipole moment for a formally neutral molecule up still further.
(more…)
References
- J. Wudarczyk, G. Papamokos, V. Margaritis, D. Schollmeyer, F. Hinkel, M. Baumgarten, G. Floudas, and K. Müllen, "Hexasubstituted Benzenes with Ultrastrong Dipole Moments", Angewandte Chemie International Edition, vol. 55, pp. 3220-3223, 2016. https://doi.org/10.1002/anie.201508249
Tags:aromatisation stabilization energy, Chemical polarity, chemical properties, Chemistry, Dipole, Electric dipole moment, Electromagnetism, energy, Moment, Nature, Physical quantities, Physics, Potential theory
Posted in Interesting chemistry | 11 Comments »
Sunday, March 18th, 2018
Around the time of the 2012 olympic games, the main site for which was Stratford in east London, I heard a fascinating talk about the “remediation” of the site from the pollution caused by its industrial chemical heritage. Here I visit another, arguably much more famous and indeed older industrial site.
(more…)
Tags:City: London, Environment, Environmental Issues, Environmental toxicology, Geography of London, industrial chemical heritage, industrial site, London Borough of Newham, London boroughs, Pollution, Province/State: Swansea, Queen Elizabeth Olympic Park, river Tawe, Stratford, Stratford London, Stratford station, Summer Olympics, the 2012 olympic games, unfortunate by-product, William Blake
Posted in Interesting chemistry | No Comments »
Wednesday, March 7th, 2018
C&EN has again run a vote for the 2017 Molecules of the year. Here I take a look not just at these molecules, but at how FAIR (Findable, Accessible, Interoperable and Reusable) the data associated with these molecules actually is.
(more…)
Tags:Carotenoids, Chemistry, Epoxides, Macrocycles, Organic chemistry, Organofluorides, PDF, Peptides, search engine, search program, search.datacite.org search engine, Technology/Internet
Posted in Chemical IT, crystal_structure_mining, Interesting chemistry | No Comments »
