The Royal Society of Chemistry historical group (of which I am a member) organises two or three one day meetings a year. Yesterday the October meeting covered (amongst other themes) the fascinating history of madder and its approximately synthetic equivalent alizarin. Here I add a little to the talk given by Alan Dronsfield on the synthesis of alizarin and the impact this had on the entire industry.
Archive for the ‘Historical’ Category
The history of Alizarin (and madder).
Thursday, October 18th, 2018Octet expansion and hypervalence in dimethylidyne-λ6-sulfane.
Tuesday, November 28th, 2017I started this story by looking at octet expansion and hypervalence in non-polar hypercoordinate species such as S(-CH3)6, then moved on to S(=CH2)3. Finally now its the turn of S(≡CH)2.‡
Twenty one years of chemistry-related Java apps: RIP Java?
Saturday, June 10th, 2017In an earlier post, I lamented the modern difficulties in running old instances of Jmol, an example of an application program written in the Java programming language. When I wrote that, I had quite forgotten a treasure trove of links to old Java that I had collected in 1996-7 and then abandoned. Here I browse through a few of the things I found.
The Chemistry Department at Imperial College London. A history, 1845-2000.
Friday, February 10th, 2017The book of the title has recently appeared giving a rich and detailed view over 417 pages, four appendices and 24 pages of photographs of how a university chemistry department in the UK came into being in 1845 and its subsequent history of discoveries, Nobel prizes and much more. If you have ever wondered what goes on in an academic department, populated by and large by very bright and clever personalities and occasionally some highly eccentric ones, then go dip into this book.
The “hydrogen bond”; its early history.
Saturday, December 31st, 2016My holiday reading has been Derek Lowe’s excellent Chemistry Book setting out 250 milestones in chemistry, organised by year. An entry for 1920 entitled hydrogen bonding seemed worth exploring in more detail here.
Bond stretch isomerism. Did this idea first surface 100 years ago?
Tuesday, February 9th, 2016The phenomenon of bond stretch isomerism, two isomers of a compound differing predominantly in just one bond length, is one of those chemical concepts that wax and occasionally wane.[1] Here I explore such isomerism for the elements Ge, Sn and Pb.
References
- J.A. Labinger, "Bond-stretch isomerism: a case study of a quiet controversy", Comptes Rendus. Chimie, vol. 5, pp. 235-244, 2002. https://doi.org/10.1016/s1631-0748(02)01380-2
I’ve started so I’ll finish. The mechanism of diazo coupling to indoles – forty (three) years on!
Thursday, December 24th, 2015
The BBC TV quiz series Mastermind was first broadcast in the UK in 1972, the same time I was starting to investigate the mechanism of diazocoupling to substituted indoles as part of my Ph.D. researches. The BBC program became known for the catch phrase I've started so I'll finish; here I will try to follow this precept with the project I started then. In 1972, one measured the rates of chemical reactions to gain insights into the transition state kinetic model. To obtain more data, we used isotopes such as 2H or 3H, together with substituents such as R-t-butyl to modify the potential energy surfaces of the reactions by inducing steric effects.[1],[2] We found that the kinetics for this reaction were actually complex‡ (in part because of pH dependence) involving a Wheland intermediate (the formation of which is shown with red curly arrows above) followed by the collapse of this intermediate to the diazo-coupled product (blue arrows). Coupling to 2-methyl indole (R=X=H, R'=Me), 2-t-butyl indole (R=H, R'=t-butyl) and 4-methyl-2-t-butyl indole (R=Me, R'=t-butyl) revealed that the kinetic isotope effects induced by replacing H by D or T were "not apparent" (i.e. close to 1), the inference being that the rate constant k1 for those systems was slower than k2; the formation of the Wheland intermediate was rate determining (the rds) for the reaction. But with 2-methyl-4,6-di-t-butyl indole (R=t-butyl, R'=Me) this changed and a deuterium isotope effect of ~7 was observed. The rate determining proton removal from the Wheland intermediate k2 was now slower than k1. With 2,4,6-tri-t-butyl indole, we ended by noting that the reaction become almost too slow to observe and furthermore was accompanied by loss of a t-butyl cation as well as a proton. At this point we attempted to infer some transition state models consistent with these observations. Note that we had relatively little data with which to derive our 3D models (one needs to define a geometry using 3N-6 variables, along with its relative energy and force constants). The text and diagram of our attempt is shown below.
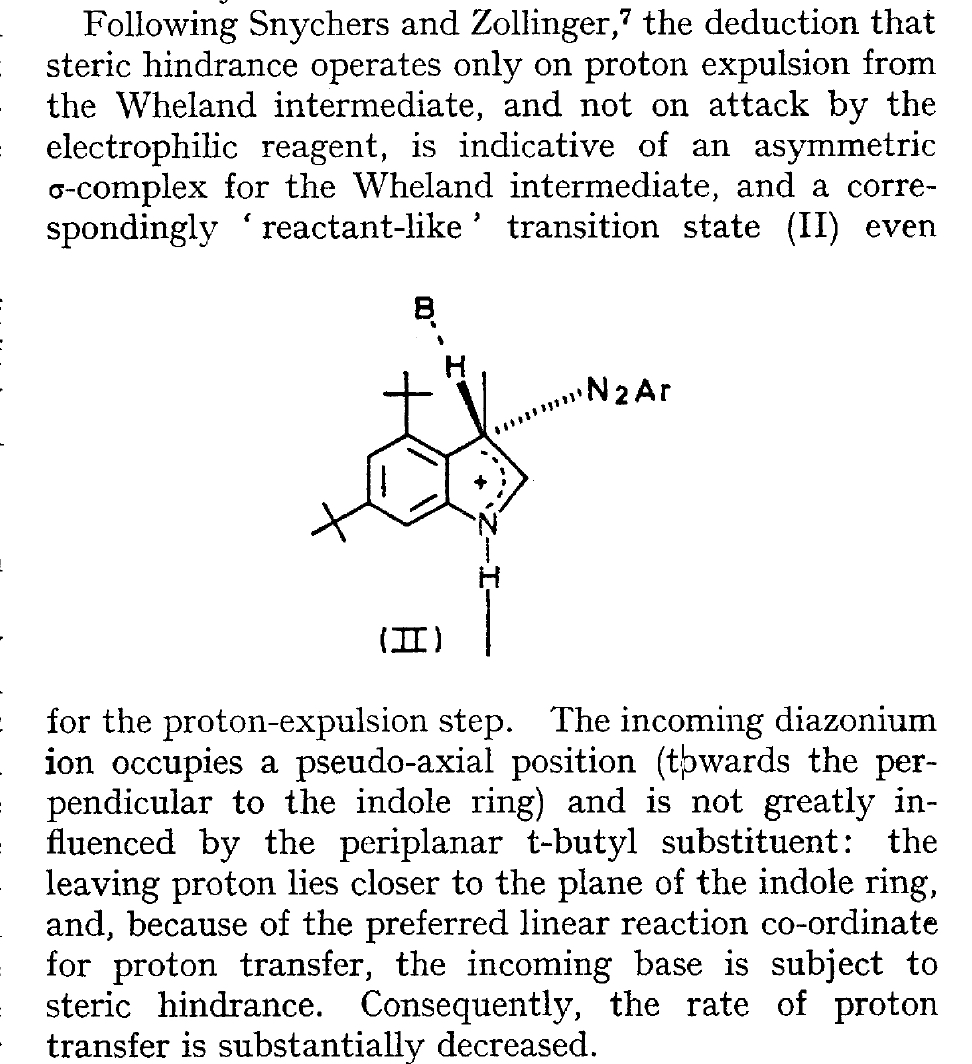 The main points of this argument were;
The main points of this argument were;
References
- B.C. Challis, and H.S. Rzepa, "The mechanism of diazo-coupling to indoles and the effect of steric hindrance on the rate-limiting step", Journal of the Chemical Society, Perkin Transactions 2, pp. 1209, 1975. https://doi.org/10.1039/p29750001209
- H.S. Rzepa, "Hydrogen Transfer Reactions Of Indoles", Zenodo, 1974. https://doi.org/10.5281/zenodo.18777