In an earlier post, I re-visited the conformational analysis of cyclohexane by looking at the vibrations of the entirely planar form (of D6h symmetry). The method also gave interesting results for the larger cyclo-octane ring. How about a larger leap into the unknown?
Let us proceed as follows. One fun game to play in chemistry is to invoke iso-electronic substitutions. In this case, we can subtitute a nitrogen and a boron atom for a pair of carbons. Thrice invoked, it leads to a molecule known as cyclotriborazane.
This species is in fact very well known, and a crystal-structure was determined some time ago (DOI: 10.1021/ja00786a022). It is worth considering some of its properties.
- The species is crystalline, and sublimes rather than melts. Contrast this with the iso-electronic cyclohexane, which melts at around 6C (itself a surprisingly high value).
- The parent H3BNH3 also has a very high melting point of > 100C, which is attributed to an extensive array of so-called dihydrogen bonds in the crystal lattice, in which a positively charged hydrogen deriving from an NH is attracted to a negatively charged hydrogen deriving from a BH. Such dihydrogen bonds have been shown to be quite strong due to this electrostatic interaction, and are responsible for the extraordinary elevation of the melting point compared to the iso-electronic ethane.
- The chair form of cyclotriborazane aligns the three hydrogens shown in either blue or red in the axial positions. The three red hydrogens might be expected to be all negatively charged, and the three blue ones positively charged. So in the chair conformation, might we expected the electrostatic repulsions between either the blue or the red hydrogens to destabilize these axial positions, and hence perhaps even destabilize the chair conformation itself?
The crystal structure however shows clearly that the chair is still the favoured conformation. Equally intriguing, one might expect the three blue hydrogens to stack up to attract electrostatically to the three red hydrogens. But you can see from the crystal packing if you activate the model below that this does not happen!
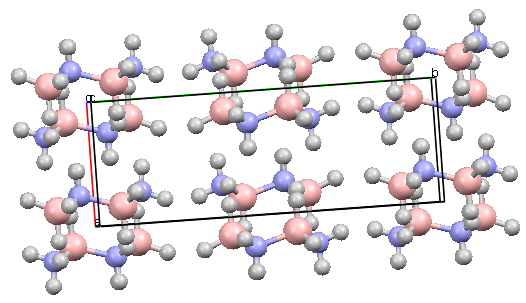
Cyclotriborazane Crystal structure. Click for 3D
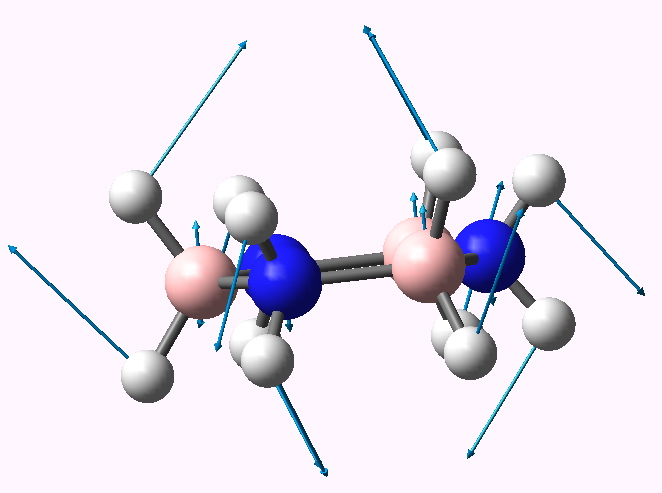
Planar cyclotriborazane distorting to chair.
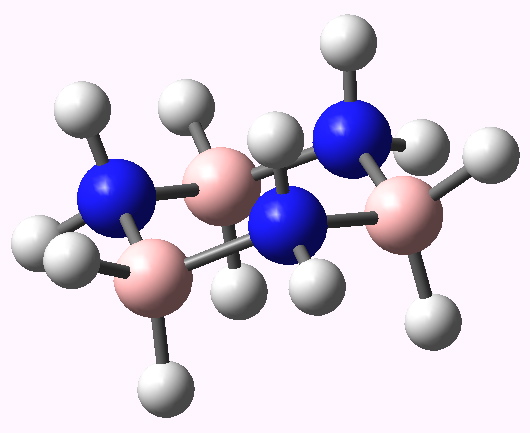
Calculated geometry of the chair form of cyclotriborazane
Tags: chair, conformational analysis, final resting energy, free energy, Interesting chemistry

[…] is negative). At the rotation angle of 0°, two H(+)…(-)H style dihydrogen bonds (see also this post) are established (these are presumed to be very attractive); at an angle of 180°, the […]