The previous post demonstrated the simple iso-electronic progression from six-coordinate carbon to five coordinate nitrogen. Here, a further progression to oxygen is investigated computationally.
The systems are formally constructed from a cyclobutadienyl di-anion and firstly the HO5+ cation, giving a tri-cationic complex. There are no examples of the resulting motif in the Cambridge structure database. A ωB97XD/Def2-TZVPP calculation (DOI: 10.14469/hpc/2350) shows it is again a stable minimum, with a Kekule mode of 1203 cm-1.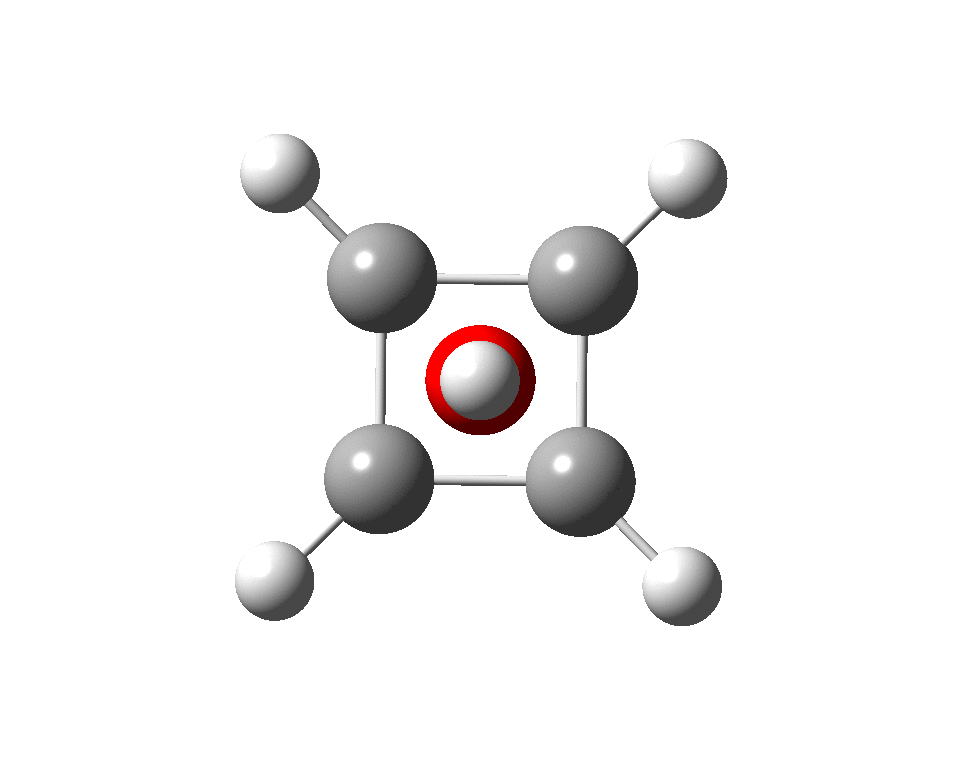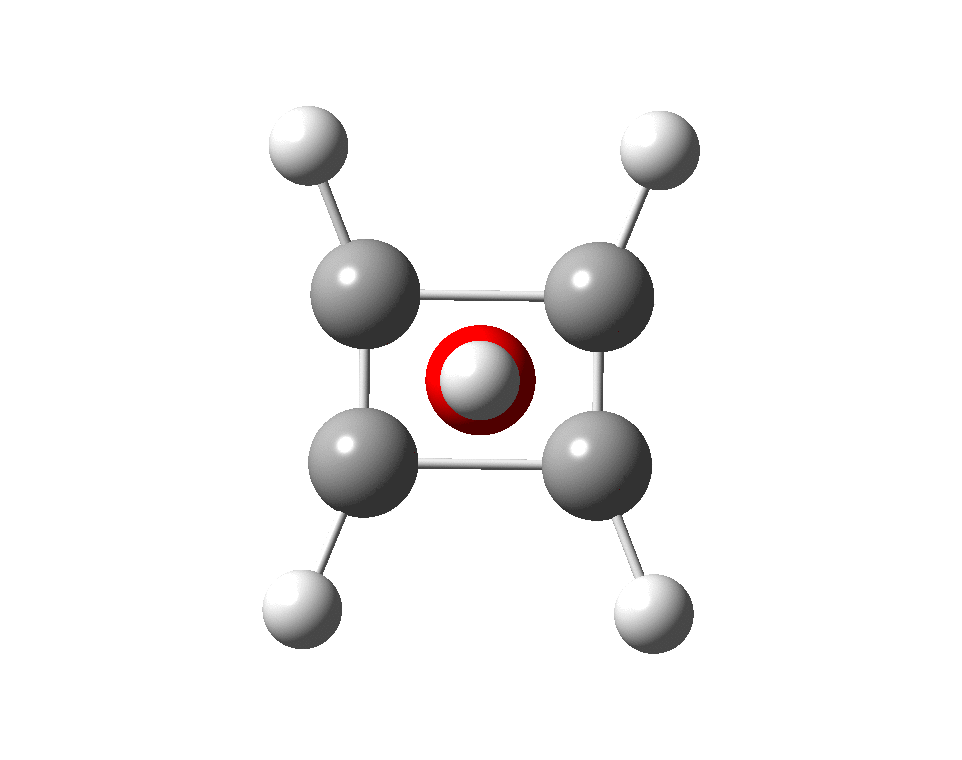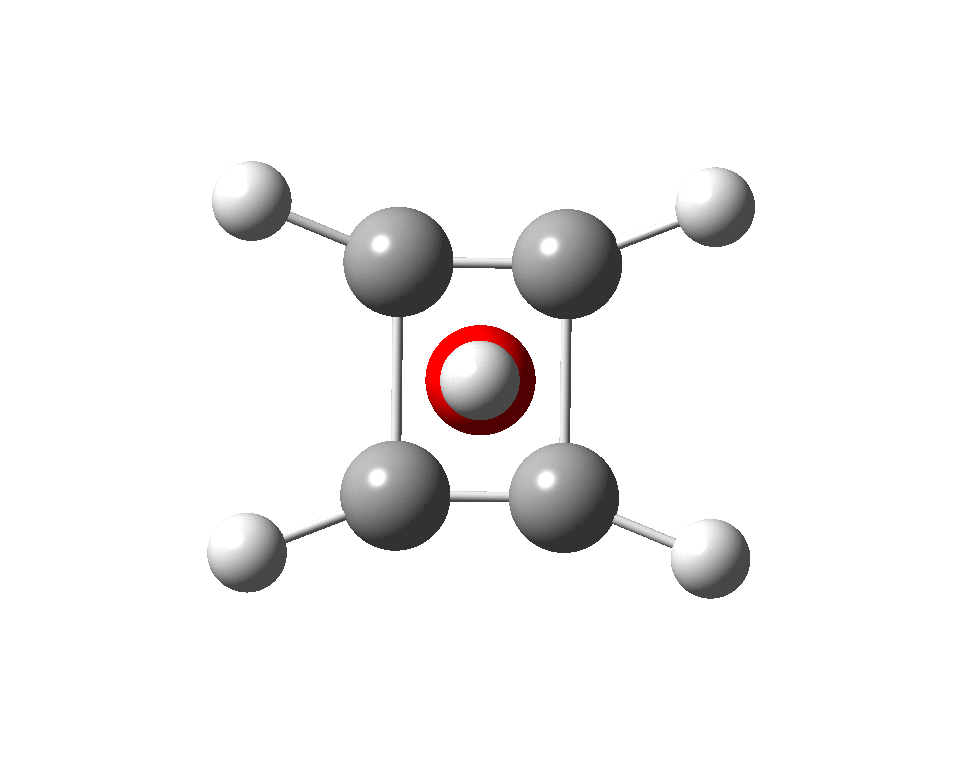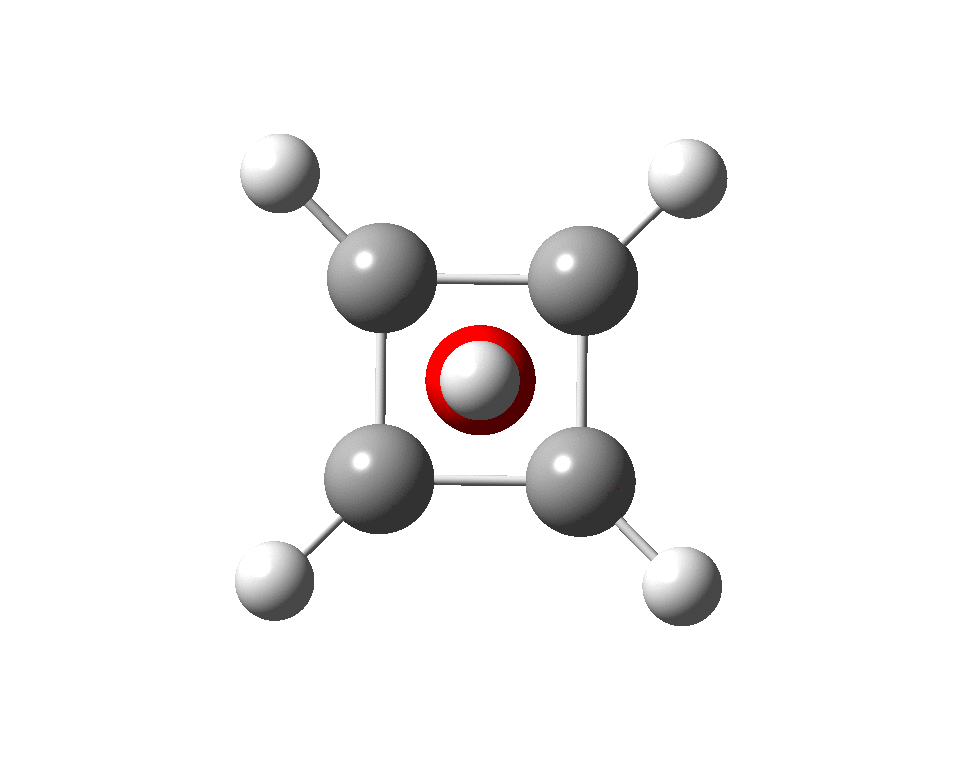
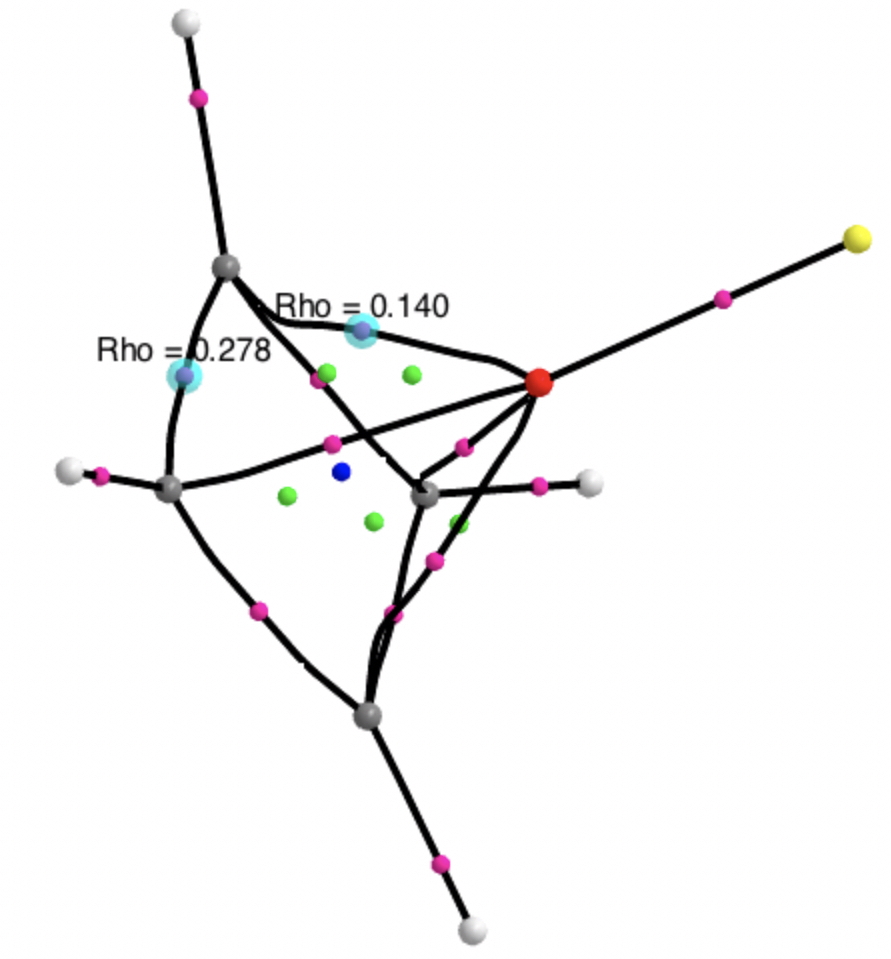
A QTAIM topological analysis of the electron density shows it differs from the nitrogen analogue in now having the ring topological feature for the basal four carbons, which in turn gives rise to a cage critical point (blue dot). The values of the electron density are lower than for N.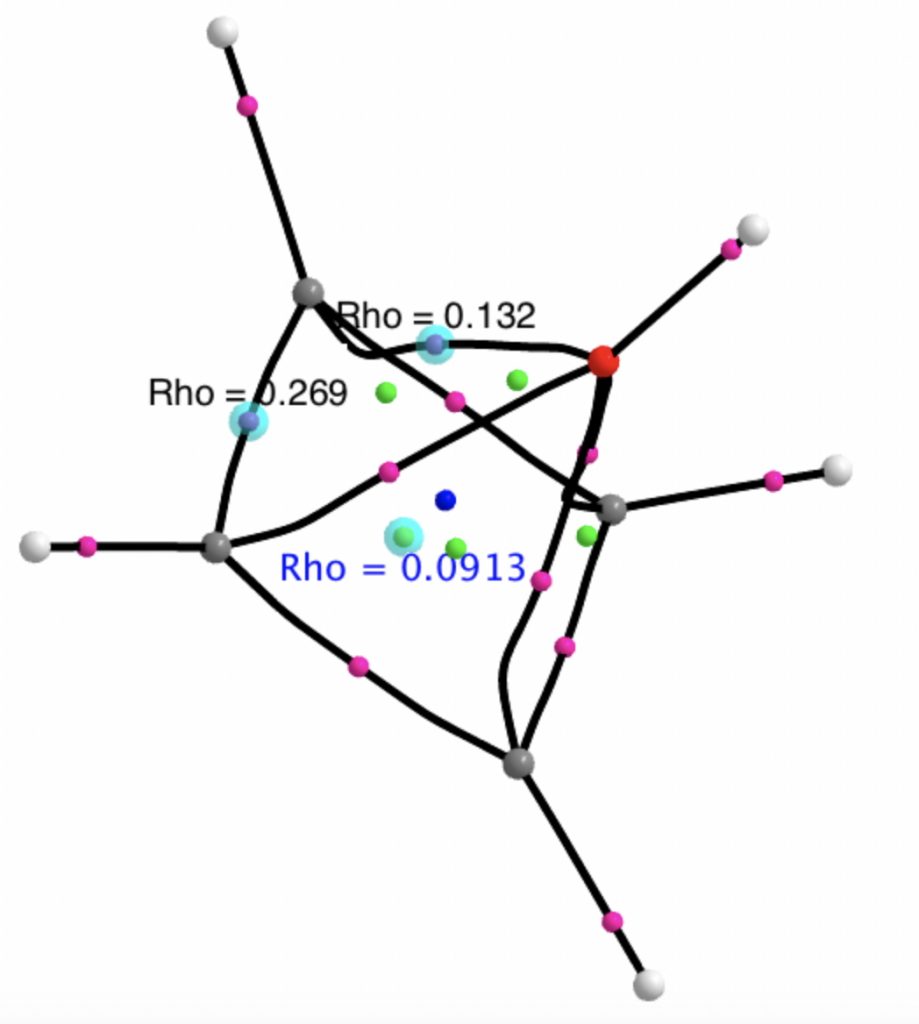
The ELF basin analysis shows the C-C bonds are regular single ones (2.01e), whereas the C-O bonds have a slightly greater electron population than the C-N bonds discussed in the previous post.
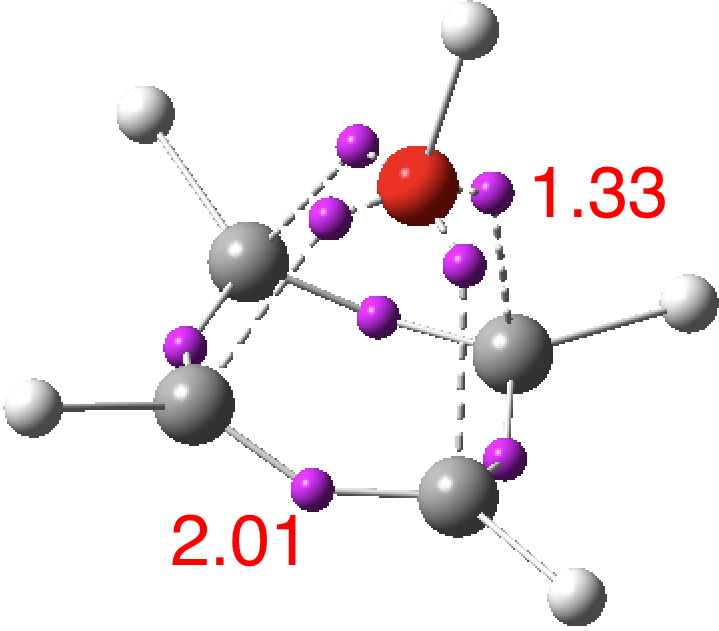
I suspect the prospects of making a stable tri-cation in such a small molecule are lower than the crystal di-cation achieved with carbon as the apical atom. But the charge can be reduced to a di-cation by replacing the HO5+ above with S–-O5+; the animation below showing the Kekule mode (1140 cm-1, DOI: 10.14469/hpc/2356).
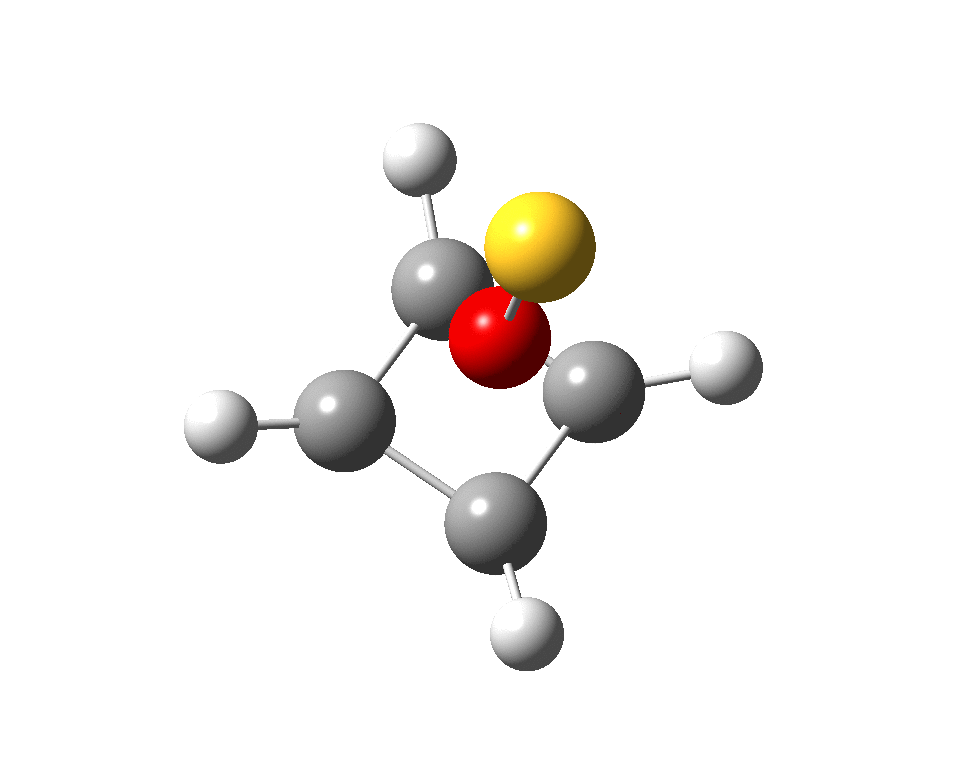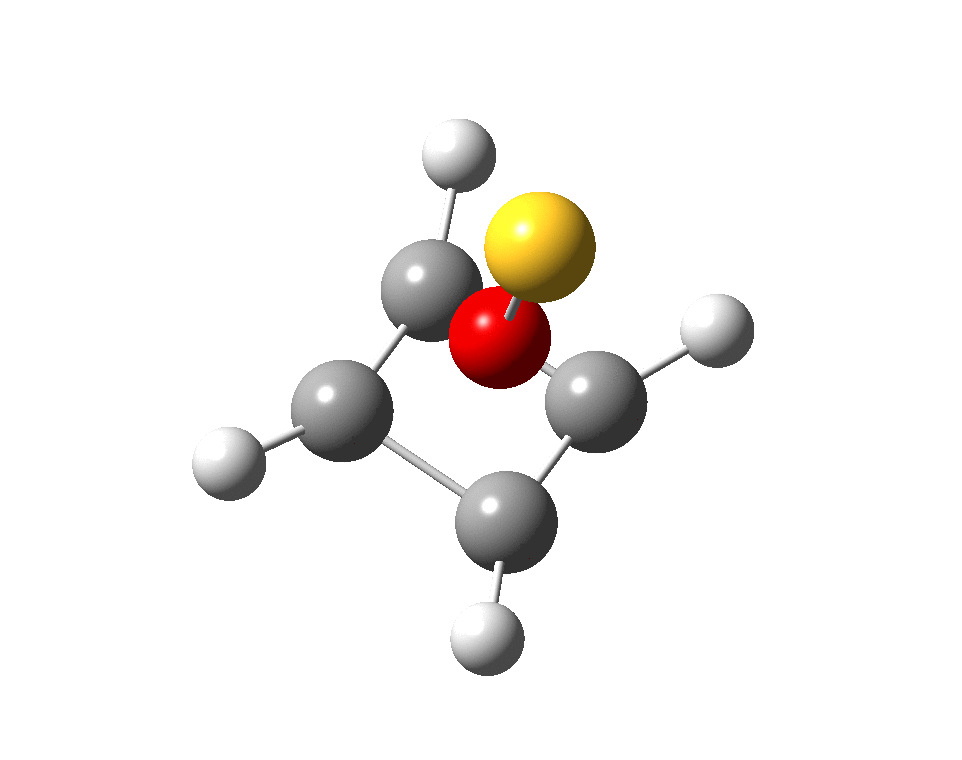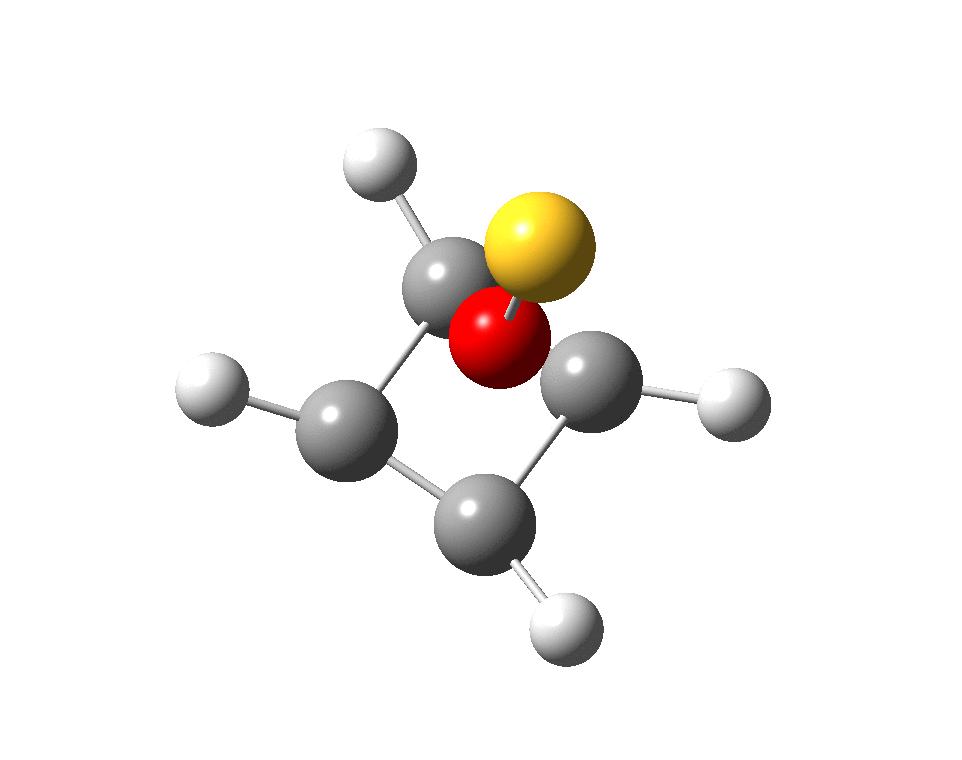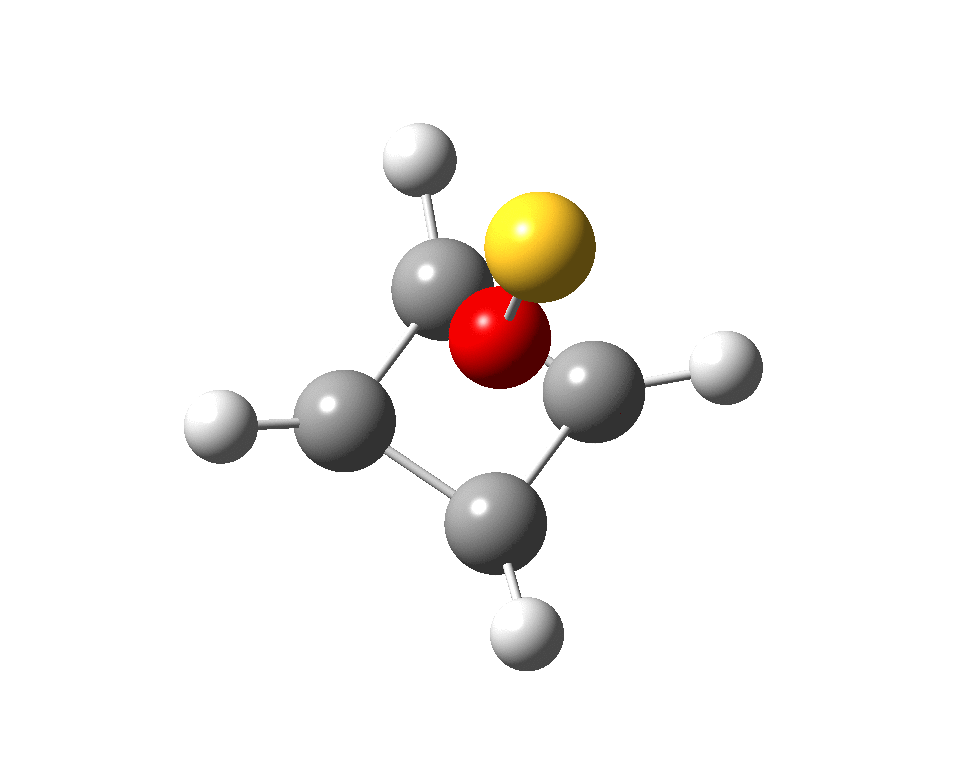
And for some (negative) loose ends.
- The P equivalent constructed from cyclobutadienyl di-anion and HP4+ is now unremarkably 5-coordinate. But in fact it is not a stable minimum (DOI: 10.14469/hpc/2357), having two negative force constants.
- as does the system from cyclobutadienyl di-anion and O=P4+(DOI: 10.14469/hpc/2358)
- and the system from cyclobutadienyl di-anion and HS5+(DOI: 10.14469/hpc/2360).
- Transposition of S/O to give O–-S5+ likewise (DOI: 10.14469/hpc/2359).
So the family of hyper-coordinate 2nd row main group elements now comprises the experimentally verified C, with N and O now open to such verification.
Tags: animation, Chemical bond, Chemistry, Matter, Nitrogen, Quantum chemistry
Would reducing the overall species to a monocation be feasible? Say, with a smallish dicarboxylato-oxo fragment such as [-OOC-CH2-CH(O[5+])-CH2-COO-][3+]?
I have no idea of the synthetic feasibility of such a species, though, or if the compensating charge would be so spread out that its stabilizing ability would be too limited.
Another option for reducing the 3+ charge is to coordinate the RO5+ to C3R33- (formally with six π electrons), giving a four-coordinate oxygen as a di-cationic molecule. The tetramethyl derivative of this (DOI: 10.14469/hpc/2363) shown below has, unfortunately, two small -ve force constants.
Here is another, rather more trivial, example of 4-coordinate oxygen (DOI: 10.14469/hpc/2364).
Another option for reducing the 3+ charge is to coordinate the RO5+ to 3CR- (formally with six π electrons), giving a four-coordinate oxygen as a di-cationic molecule. The tetramethyl derivative of this (DOI: 10.14469/hpc/2363) shown below has, unfortunately, two small -ve force constants.