More than 100 years ago, before the quantum mechanical treatment of molecules had been formulated, G. N. Lewis proposed[cite]10.1021/ja02261a002[/cite] a simple model for chemical bonding that is still taught today. This is the idea of the three categories of bond we know as single, double and triple, comprising respectively two, four and six shared electrons each, at least for the very common carbon-carbon bond. A little more than a decade ago, this was extended upwards to the eight-electron quadruple bond.[cite]10.1038/nchem.1263[/cite]. Now, at the other extreme of downwards, a molecule has been characterised in the solid state with a one-electron C-C bond.[cite]10.1038/s41586-024-07965-1[/cite] In this sub-two-electron region, bonds such as hydrogen bonds have long been recognised and they form part of a class of “weak” bonding known instead as exhibiting “non-covalent-interactions” or NCI. But specifically a one-electron carbon-carbon bond stands apart from these weaker types and so it is certainly news when one such is reported and characterised in the crystalline state by x-ray diffraction.
To start the investigation, a search of the crystal structure database was performed using the following more general query of the structure above. The central C-C bond (in green below) was not added, leaving the two carbons as 3-coordinate.
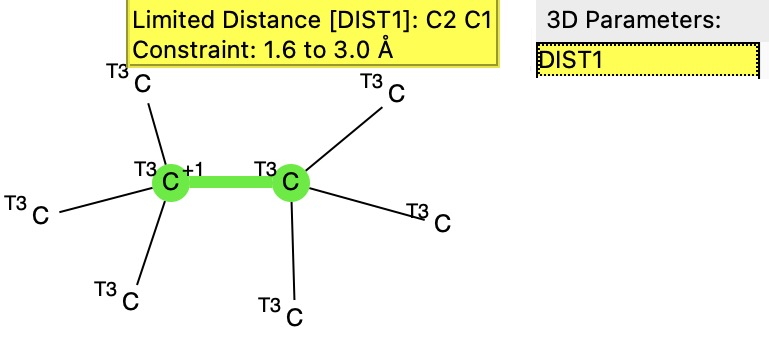
This resulted in 10 hits, all revealed as dications, with the central C-C distance ranging from 2.8Å to 3.0Å. So the unique feature of this new report is that they were able to find a system where oxidation did not proceed directly to the dication, but stopped at the 1-electron level to give a radical cation instead. This new structure poses a bit of a quandry for the curators of the CSD. The index for this database is built on the basis of whether any two atoms in a molecule are connected by a “bond”, and the allowed values for bonds range from single to quadruple, with various intermediate descriptions (such as aromatic) and finally “any”. This latter basically means any of the previous, but what I am pretty certain of is that it does not mean “one-electron”, or “half”. The new compound has not yet been indexed in my current version of the CSD, so this presumption is not yet tested.
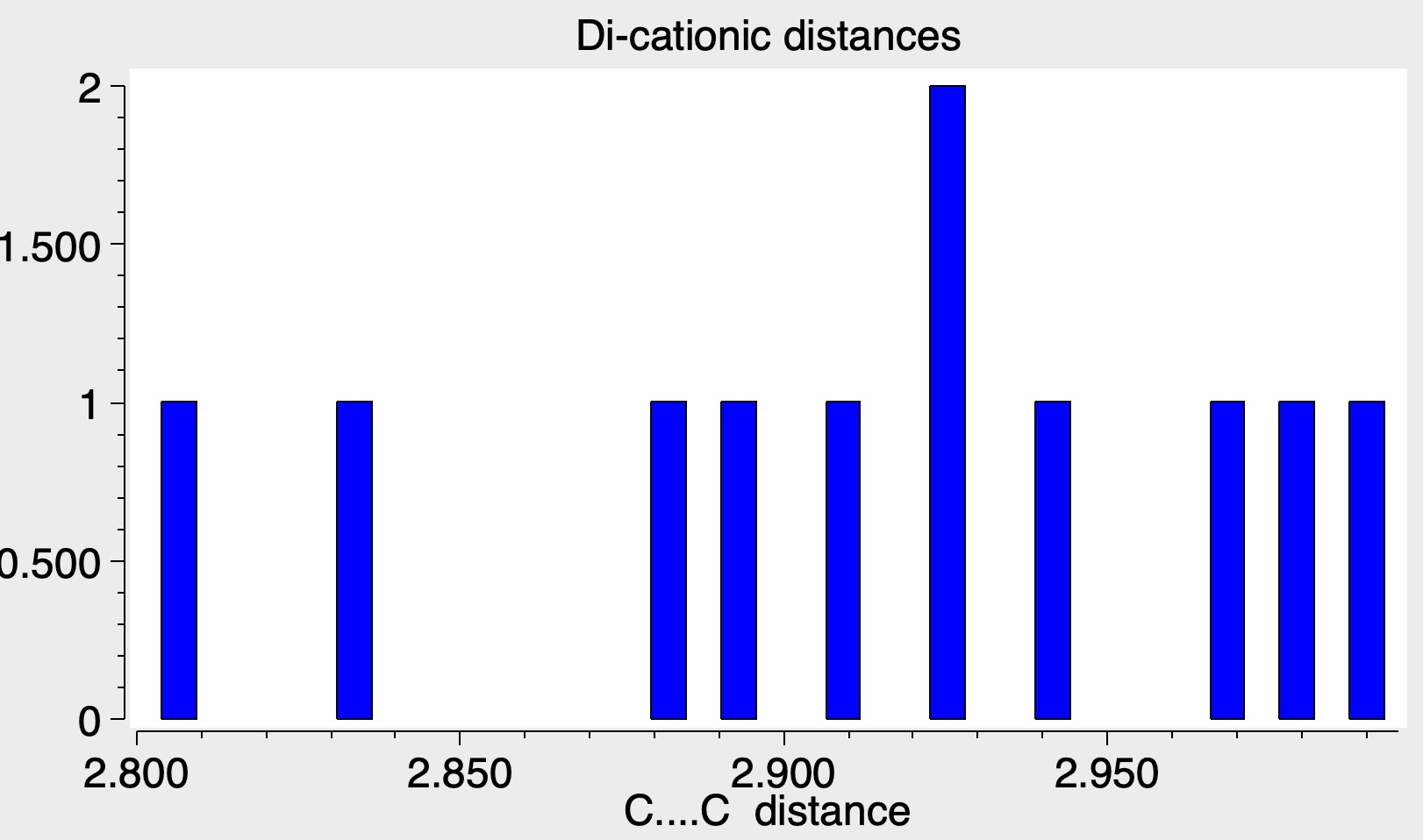
The authors[cite]10.1038/s41586-024-07965-1[/cite] did also make the dication and they report a length of 3.03Å for this species, broadly in accord with the range shown above and a reduced value of 2.92Å for the radical cation (Δr 0.11Å). This is quite a small contraction induced by the formation of the one-electron bond, which is already hinting that it might actually be a weak bond.
Next, I proceeded by performing my own DFT calculations on these species, at the ωB97XD/Def2-TZVPP level.(FAIR data DOI: [cite]10.14469/hpc/14642[/cite]) At this level the di- and monocationic C-C bond lengths came out as 3.075Å and 2.867Å (Δr 0.21Å), a slightly larger contraction than that reported, but still representing a weak bond.
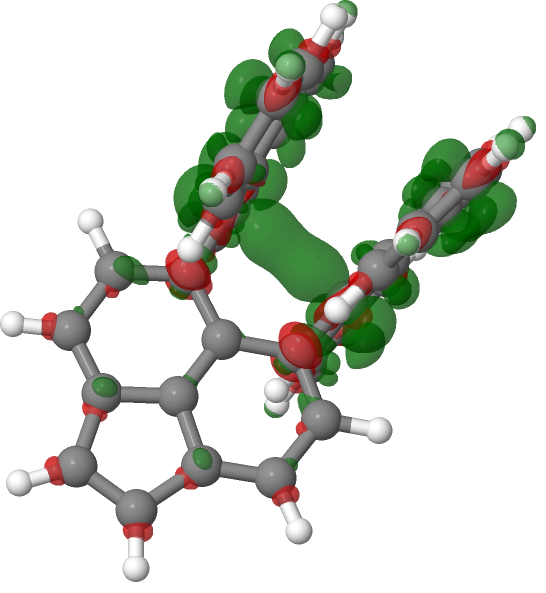
With wavefunctions now available for the species, I decided to inspect the electron densities. This was calculated at the geometry of the radical cation, and then at the same geometry, the dication was calculated and the two electron densities subtracted. The resulting density surface, representing one electron is shown below. As expected, the most significant feature occurs in the C-C region, but quite a lot of this one electron is distributed around the aromatic rings (I must find out how to integrate regions!). So already we see that this “1-electron” bond is in fact only a fraction of one electron. Again an indication that it is a weak bond.
A procedure often used to identify weak bonds is called NCI, or noncovalent-interactions.[cite]10.1021/ja100936w[/cite] These are by definition interactions weaker than the single bonds, often being hydrogen bonds and other unusual interactions such as a π-π stacking region (rather than a bond). So here, we see that below the single bond type, we get a continuum of interactions rather than bonds as such. The resulting NCI analysis is shown below for firstly the radical cation and then the di-cation at the same geometry.
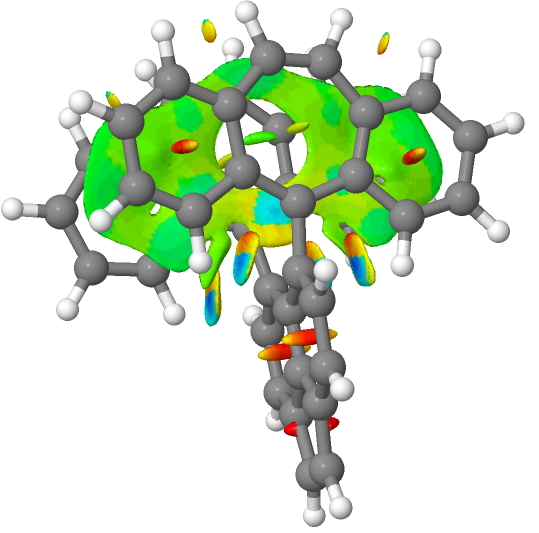
The colour coding in the NCI surface analysis above means that dark blue are strong non-covalent interactions such as hydrogen bonds, paler blue or cyan areas are weaker ones and green is weaker still and typical of π-π stacking regions rather than bonds between two atoms. These are all deemed stabilising, whereas orange and red regions are destabilising. Click on the image above to inspect the full three dimensional surface of this NCI function and you will find the π-π stacking features, but also three cyan regions. Enclosed by two of the cyan regions are dark blue ones, whilst the third cyan region contains only a small blue part. This third cyan region is indeed in the C-C one-electron bond region, but using this analysis it emerges as only a “weak” interaction.
But a surprise! The two dark blue regions, deemed strong “interactions” are between a C-H of an aryl group and the two carbon atoms shown with blue dots in the diagram above and these are apparently more stabilizing than the one-electron C-C “bond”. Should they not also be bonds then?
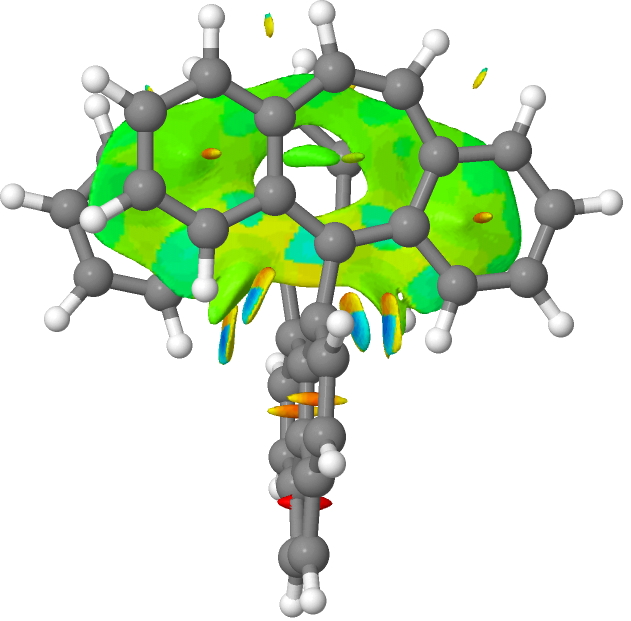
The plot above is for the di-cation at the radical cation geometry. It emerges as very similar to the radical cation itself, although the C-C cyan NCI region is less intense than that for the latter and now contains little trace of the dark blue inner core.
We might conclude from this inspection of the newly reported molecule containing a one-electron C-C bond, is that it probably belongs to the class known as an “interaction” rather than an actual bond. Even as an interaction, it is not particularly strong – in part this is probably because only a proportion of that one electron is actually located in the C-C region, with the rest being distributed around the aromatic rings. However, I rather suspect that despite it resembling an interaction, it will no doubt become known as a bond!
Added in response to comment
Below is shown the Laplacian of the electron density (a definition can be found at eg [cite]10.59350/bk5zm-6rk67[/cite]). Negative values of the Laplacian appear here in purple and positive values in orange (contour value 0.125 a.u). The regular C-C bonds are all enclosed in a negative region of the Laplacian, whilst the one-electron C-C bond lies in the orange region.
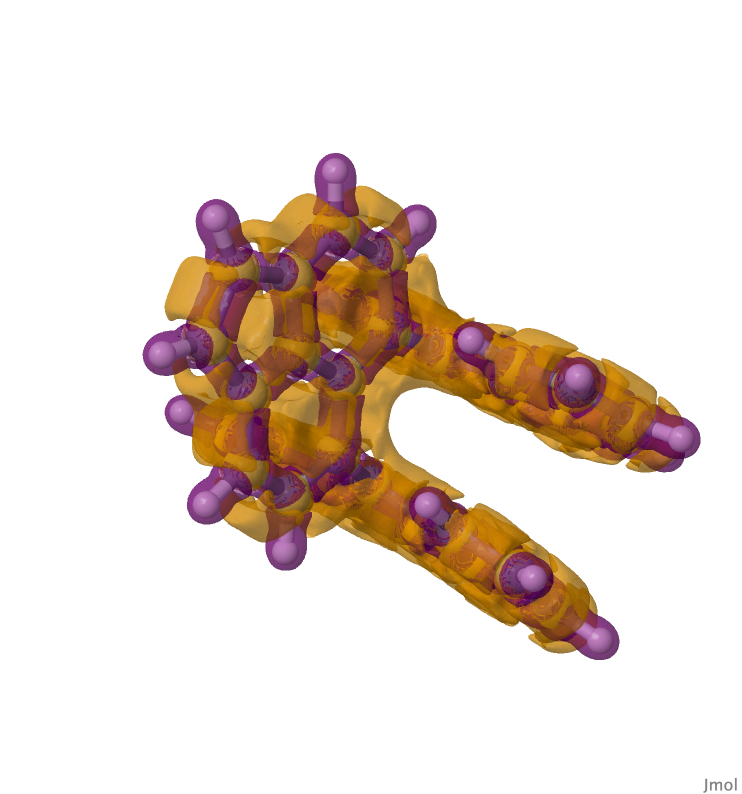
Hello!
I read in the support information of the article where they show QTAIM results that the BCP’s Laplacian between those two carbons is positive (covalent bonds have it negative) in line with your NCI analysis that between those two C-C there is no one electron covalent bond.
Yes, the NCI function also includes calculation of the Laplacian. I will add a 3D model of the Laplacian to the blog.
Laplacian (contoured at 0.125 au) added.