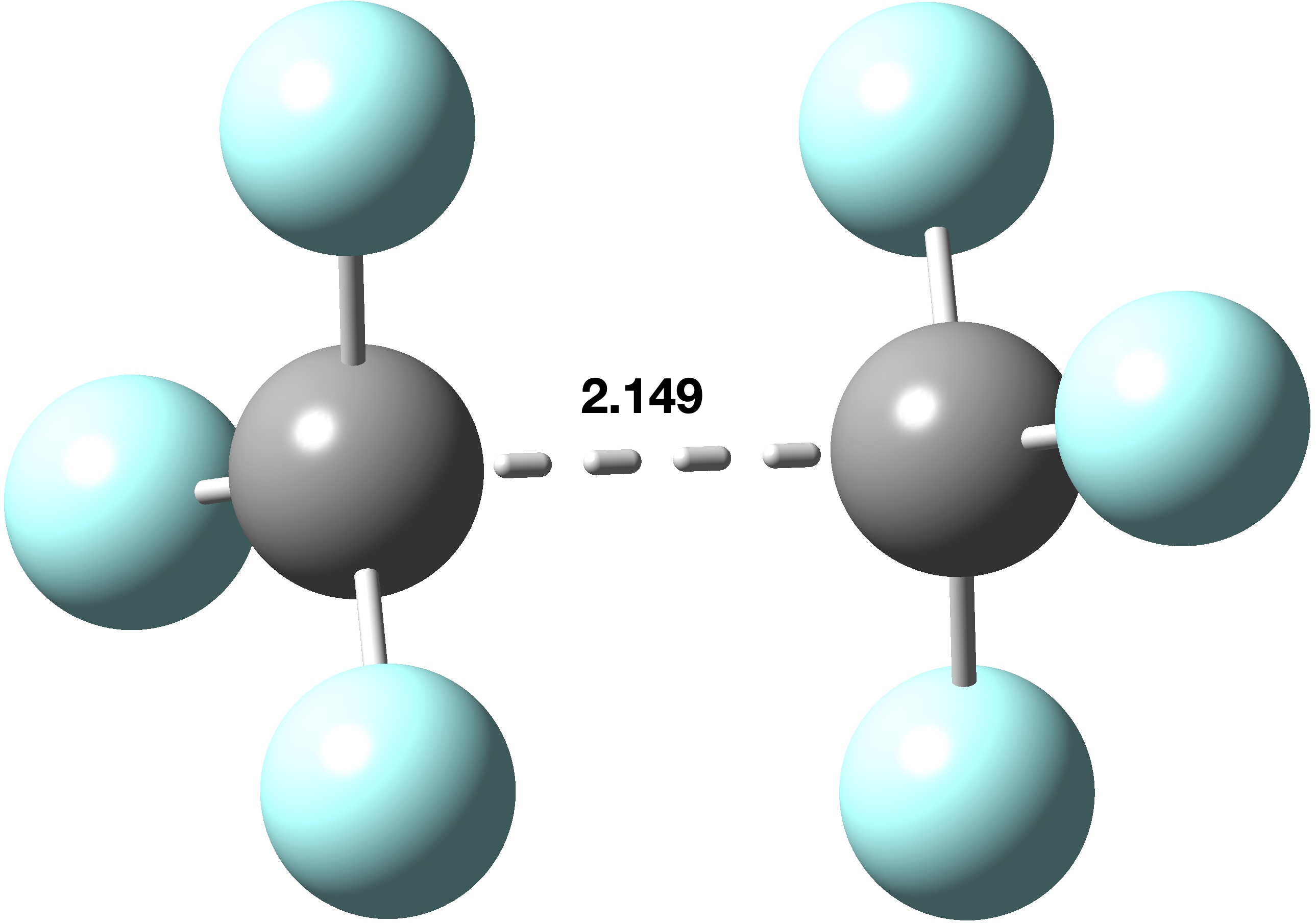
In the previous post, I looked[cite]10.59350/xp5a3-zsa24[/cite] at the recently reported[cite]10.1021/ja02261a002[/cite] hexa-arylethane containing a carbon-carbon one-electron bond, its structure having been determined by x-ray diffraction (XRD). The measured C-C bond length was ~2.9aÅ and my conclusion was that the C…C region represented more of a weak “interaction” than of a bond as such. How about a much simpler system, hexafluoroethane? Here, the two-electron C-F bonds are much lower in energy than the C-C bond, so when the molecule is ionised, it escapes from the C-C bond rather than any of the C-F bonds. The below is the structure computed at the ωB97XD/Def2-TZVPP level, revealing a much shorter C-C bond of 2.149Å. The computed C-C stretching vibrational frequency is 179 cm-1 (FAIR data DOI: [cite]10.14469/hpc/14642[/cite])
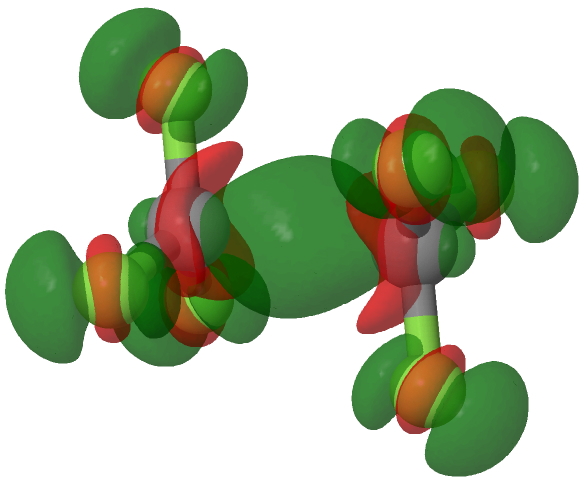
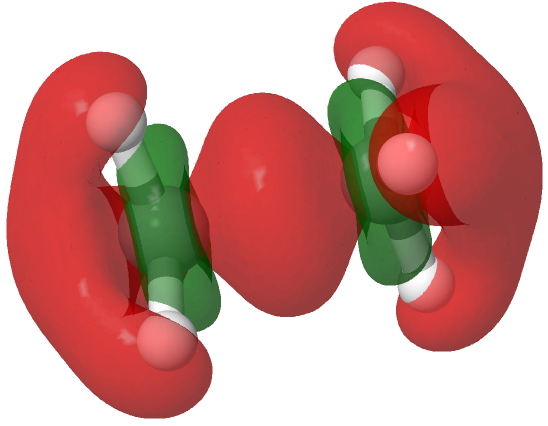
An electron density difference map, obtained by subtracting the computed density of the dication from that of the radical cation at the geometry of the former is shown below, confirming that the electron has been removed from the C-C region, with a smaller removal from the C-F bonds.
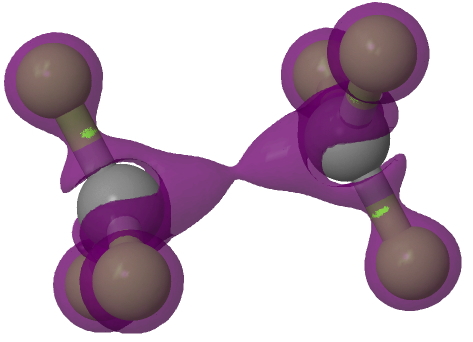
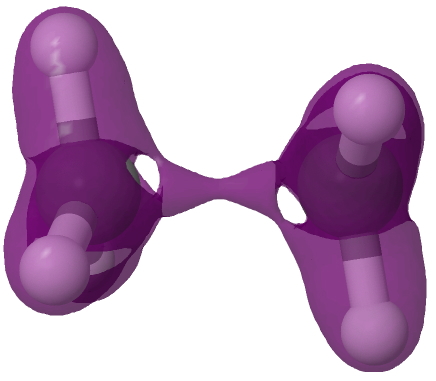
The Laplacian of the electron density is shown below contoured for negative values of this function. Unlike the previous molecule, this now has a (small) negative value along the C-C region (contour -0.001).
A calculation of the NCI surface gave a null result! The parameters for computing a non-covalent analysis are thus: [0.5 1 0.0005 0.05 0.95 1.00], being the ones used in the previous analysis. The value of 0.05 is the density cutoff used to remove covalent density and using this value, no non-covalent features are detected. Or, put another way, only covalent features are present, as supported by the -ve Laplacian noted above.
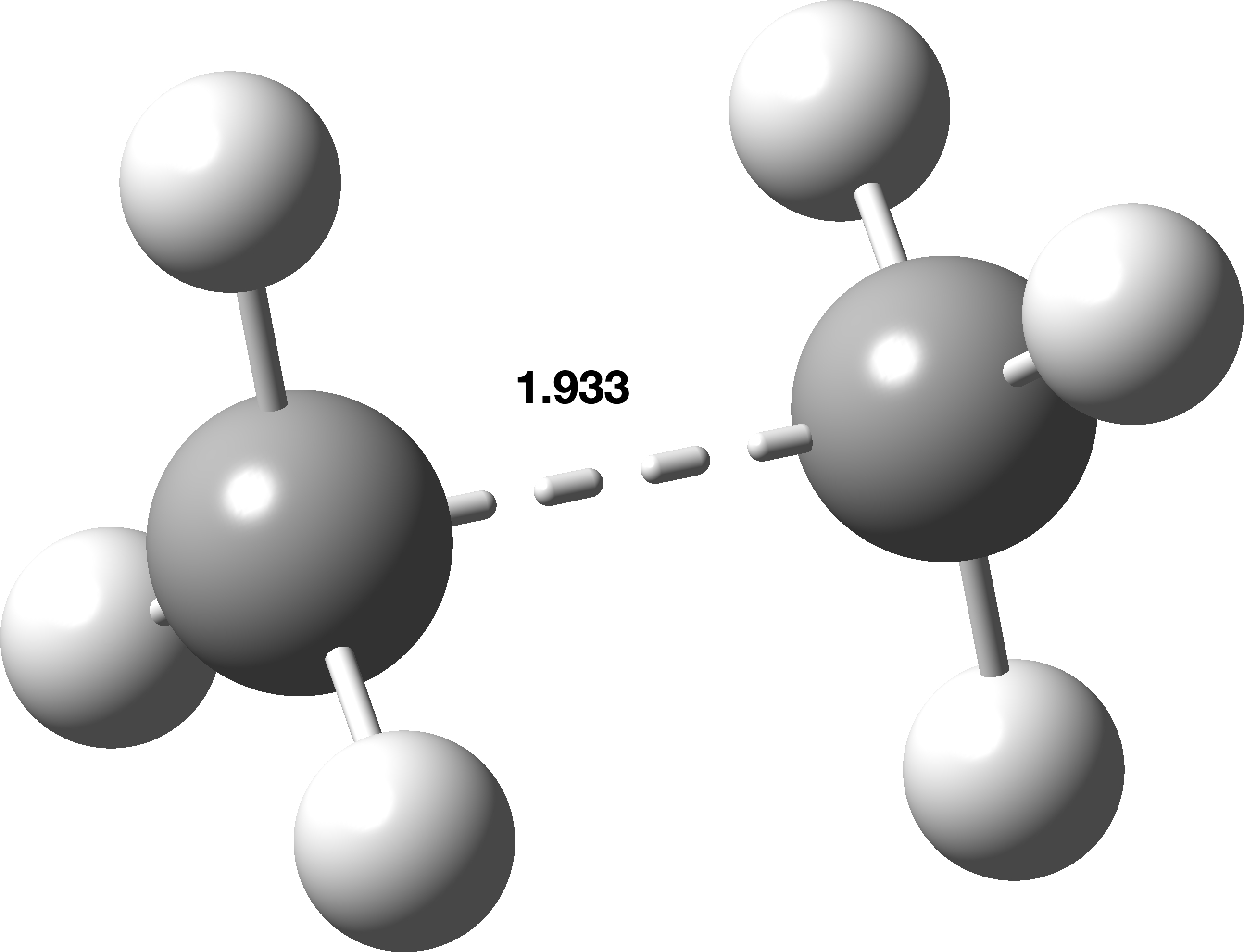
Whilst C2F6+. cannot be claimed to be typical of a molecule with a hypothetical “pure” one-electron C-C bond, it is certainly very different from the previous example.[cite]10.59350/xp5a3-zsa24[/cite],[cite]10.1021/ja02261a002[/cite] Time to go all the way and try ethane itself, C2H6+.. Again the same behavour is seen, whilst the calculated C-C length reduces to 1.933Å. The C-C stretching vibrational frequency is elevated to 477 cm-1. We might take these last values as the natural ones for a one-electron C-C bond?
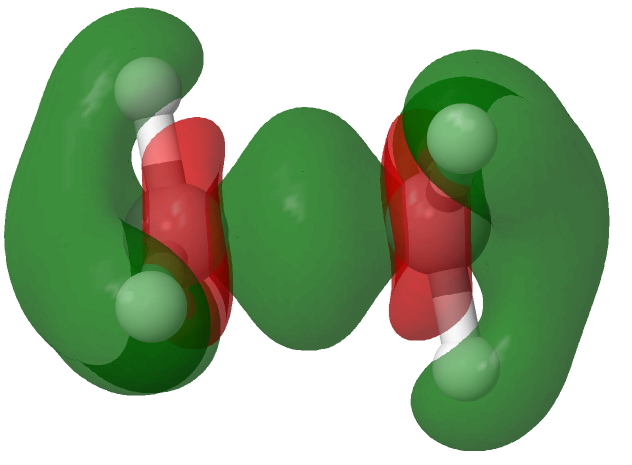
This alternative subtraction involves the density difference between neutral ethane and its radical cation. The result is essentially the same.
So these two ethane derivatives add some further context to the properties of a one-electron C-C bond. We have seen them range from a low of ~1.9Å to a high of ~2.9Å This variation of around 1Å as a function of the substituents on the two carbons must be the largest ever seen for any kind of bond!
For curiosity’s sake, I investigated the corresponding neutral-but-isoelectronic compounds in which one carbon is replaced by a boron atom. I’m using a free online service, so I can’t simulate these in the ωB97XD/Def2-TZVPP model. OTOH, I can simulate them in ωB97XD/6-31G(d); hopefully the difference is immaterial.
H₃B-CH₃ is clearly bonded, in the sense that there is net σ occupancy between B and C. In fact, there is a full σ bond, 1.57 Å long and vibrating at 893/cm. The electron is missing elsewhere: two hydrogens on the boron approach each other to form (per natural bond analysis) a 3c-3e bond with boron.
In contrast, F₃B…CF₃ is unbound, because the only filled orbital between B and C is an unmixed C lone pair (or, since the orbital is half-filled, lone electron).
Probably there exist ligands of intermediate electronegativity, such that the compound as a whole is a single bound radical, but the missing electron is withdrawn from the C-B bond. I’m bumping up against the free service’s computation limits, so I shan’t look for them (in a more physical model than RHF).
OK, so I thought of two more examples that I could investigate in ωB97XD/6-31G(d).
NC-BN has a net bond 1.49 Å long and oscillating at 741/cm. But again the central bond has a full 2e-; the electron is lost from B-N instead.
In ωB97XD/6-31G(d), NC-CO has a negative-frequency oscillation in which CN and CO spin in opposite directions, so I didn’t investigate further.
Thanks for those Jacob. It shows how a simple molecule can trigger such lateral explorations.
[…] in which a molecule isoelectronic woith ethane, namely the methyl-λ1-borane radical (H3B-CH3) was proposed by Jacob. The optimised structure at the ωB97XD/6-31G(d) level exhibited a B-C bond length of 1.57Å, with […]
Jacob, I took a look at H₃B-CH₃. Clearly at least two structural isomers are possible, the one you describe and another I located. See https://doi.org/10.59350/sjrdz-3cm71 for details of this latter, which is in fact similar to ethane and hexafluoroethane, but with an even shorter C-B bond.
I have also added the radical anion of diborane, isoelectronic with the radical cation of ethane. It has its own peculiar features, and in particular the electron density difference map between it and neutral diborane shows that the electron just not just come from the B-B bond but other fascinating regions as well. The concept of a two-electron bond has dissolved!