September 6th, 2014
In the previous posts, I explored reactions which can be flipped between two potential (stereochemical) outcomes. This triggered a memory from Alex, who pointed out this article from 1999[1] in which the nitrosonium cation as an electrophile can have two outcomes A or B when interacting with the electron-rich 2,3-dimethyl-2-butene.  NMR evidence clearly pointed to the π-complex A as being formed, and not the cyclic nitrosonium species B (X=Al4–). If you are wondering where you have seen an analogy for the latter, it would be the species formed when bromine reacts with an alkene (≡ Br+, X=Br– or Br3–). The two structures are shown below[1]
NMR evidence clearly pointed to the π-complex A as being formed, and not the cyclic nitrosonium species B (X=Al4–). If you are wondering where you have seen an analogy for the latter, it would be the species formed when bromine reacts with an alkene (≡ Br+, X=Br– or Br3–). The two structures are shown below[1] 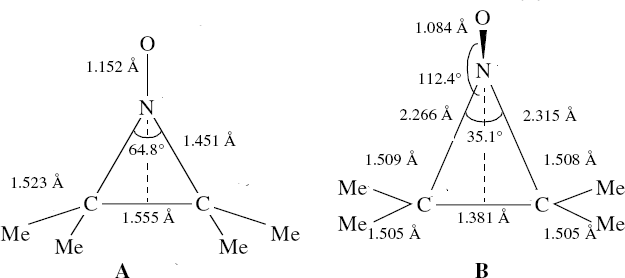 Since the topic that sparked this concerned pericyclic reactions, it seemed possible that if it had been formed, species B would immediately undergo a pericyclic electrocyclic reaction to form the rather odd-looking cation C, which might then be trapped by eg X(-) to form the nitrone D. So this post is an exploration of what happens when X-NO (X= CF3COO, trifluoracetate) interacts with 2,3-dimethyl-2-butene, as an illustration of what can be achieved nowadays from about 2 days worth of dry-lab computation as a prelude to e.g. an experiment in the wet-lab (it would take a little more than two days to achieve the latter I suspect). Hence computationally directed synthesis. The model is set up as ωB97XD/6-311G(d,p)/SCRF=chloroform. A transition state is located[2] and the resulting IRC (below) [3] does not quite have the outcome the above scheme would suggest.
Since the topic that sparked this concerned pericyclic reactions, it seemed possible that if it had been formed, species B would immediately undergo a pericyclic electrocyclic reaction to form the rather odd-looking cation C, which might then be trapped by eg X(-) to form the nitrone D. So this post is an exploration of what happens when X-NO (X= CF3COO, trifluoracetate) interacts with 2,3-dimethyl-2-butene, as an illustration of what can be achieved nowadays from about 2 days worth of dry-lab computation as a prelude to e.g. an experiment in the wet-lab (it would take a little more than two days to achieve the latter I suspect). Hence computationally directed synthesis. The model is set up as ωB97XD/6-311G(d,p)/SCRF=chloroform. A transition state is located[2] and the resulting IRC (below) [3] does not quite have the outcome the above scheme would suggest. 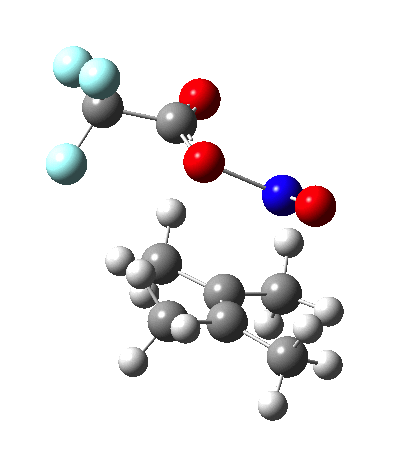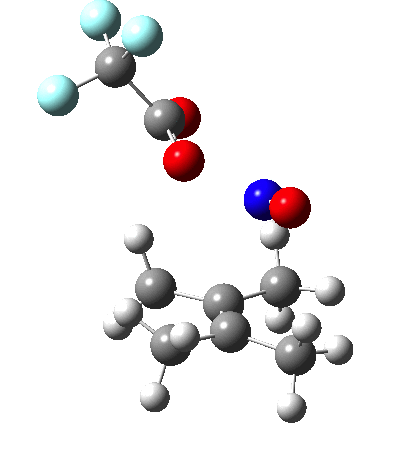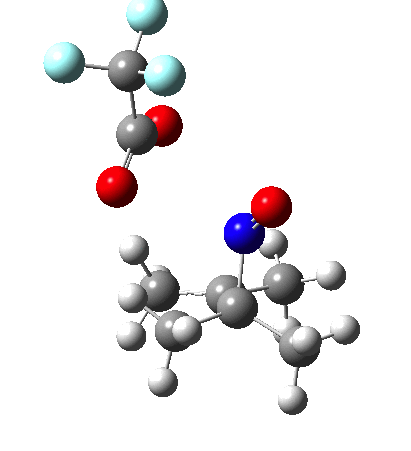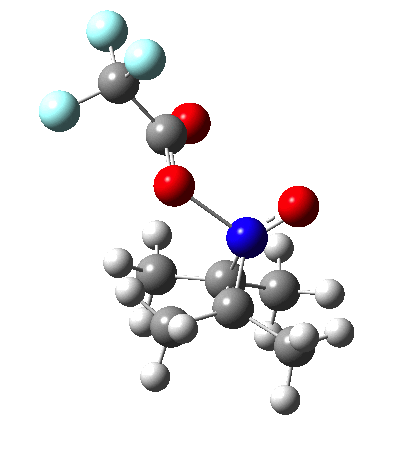

 Neither A nor B is formed; instead it is the tetrahedral species E, which is ~15 kcal/mol endothermic.
Neither A nor B is formed; instead it is the tetrahedral species E, which is ~15 kcal/mol endothermic.  I should immediately point out that this is not inconsistent with the formation of A as previously characterised[1]. That is because this experiment was conducted with a non-nucleophilic counter-anion (X=Al4–), whereas in the computational simulation above, we have a nucleophilic anion (X= CF3CO2–). What a difference the inclusion of a counter-ion in the calculation can have! The barrier however (~35 kcal/mol) is a little too high for a facile thermal reaction. In the second of this two-stage reaction, E now ring-opens to form the anticipated D[4] with quite a small barrier of ~6 kcal/mol, but a highly exothermic outcome. I ask this question about it; can this still be described as a pericyclic process? (there is some analogy to the electrocyclic ring opening of a cyclopropyl tosylate).
I should immediately point out that this is not inconsistent with the formation of A as previously characterised[1]. That is because this experiment was conducted with a non-nucleophilic counter-anion (X=Al4–), whereas in the computational simulation above, we have a nucleophilic anion (X= CF3CO2–). What a difference the inclusion of a counter-ion in the calculation can have! The barrier however (~35 kcal/mol) is a little too high for a facile thermal reaction. In the second of this two-stage reaction, E now ring-opens to form the anticipated D[4] with quite a small barrier of ~6 kcal/mol, but a highly exothermic outcome. I ask this question about it; can this still be described as a pericyclic process? (there is some analogy to the electrocyclic ring opening of a cyclopropyl tosylate). 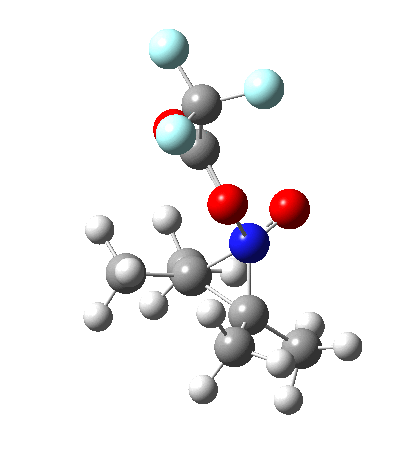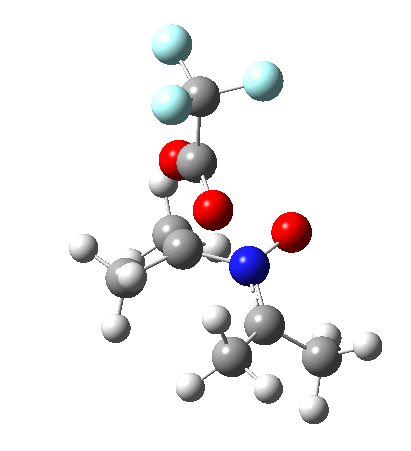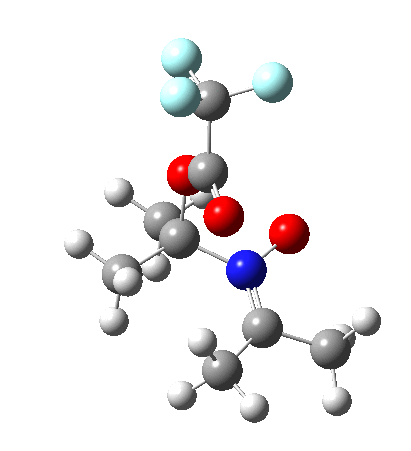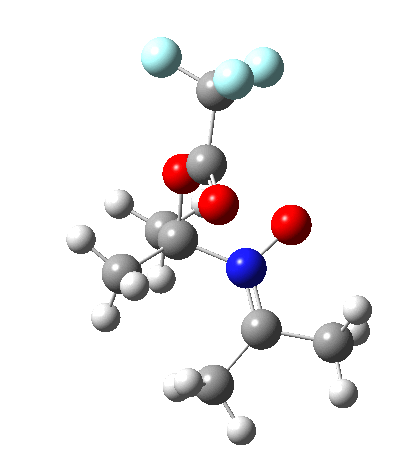
 So what are the conclusions? Well, because of the rather high initial barrier, the alkene will need activation (by electron donating substituents, perhaps OMe) for the reaction to become more viable. But if it works, it could be an interesting synthesis of nitrones (I have not yet searched to find out if the reaction is actually known).
So what are the conclusions? Well, because of the rather high initial barrier, the alkene will need activation (by electron donating substituents, perhaps OMe) for the reaction to become more viable. But if it works, it could be an interesting synthesis of nitrones (I have not yet searched to find out if the reaction is actually known).
References
- G.I. Borodkin, I.R. Elanov, A.M. Genaev, M.M. Shakirov, and V.G. Shubin, "Interaction in olefin–NO+ complexes: structure and dynamics of the NO+–2,3-dimethyl-2-butene complex", Mendeleev Communications, vol. 9, pp. 83-84, 1999. https://doi.org/10.1070/mc1999v009n02abeh000995
- H.S. Rzepa, "C8H12F3NO3", 2014. https://doi.org/10.14469/ch/24979
- H.S. Rzepa, "Gaussian Job Archive for C8H12F3NO3", 2014. https://doi.org/10.6084/m9.figshare.1162797
- H.S. Rzepa, "Gaussian Job Archive for C8H12F3NO3", 2014. https://doi.org/10.6084/m9.figshare.1162676
Tags: CF 3, CF 3 CO, COO, simulation
Posted in pericyclic, reaction mechanism | 2 Comments »
August 18th, 2014
This post, the fifth in the series, comes full circle. I started off by speculating how to invert the stereochemical outcome of an electrocyclic reaction by inverting a bond polarity. This led to finding transition states for BOTH outcomes with suitable substitution, and then seeking other examples. Migration in homotropylium cation was one such, with the “allowed/retention” transition state proving a (little) lower in activation energy than the “forbidden/inversion” path. Here, I show that with two electrons less, the stereochemical route indeed inverts. First, a [1,4] alkyl shift with inversion at the migrating carbon (ωB97XD/6-311G(d,p)/SCRF=chloroform); as a four-electron process, this is the “allowed” route.[1]
First, a [1,4] alkyl shift with inversion at the migrating carbon (ωB97XD/6-311G(d,p)/SCRF=chloroform); as a four-electron process, this is the “allowed” route.[1] 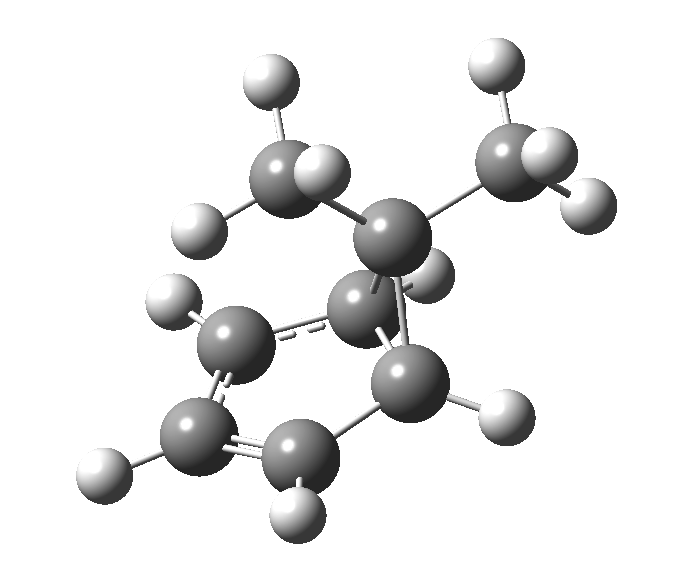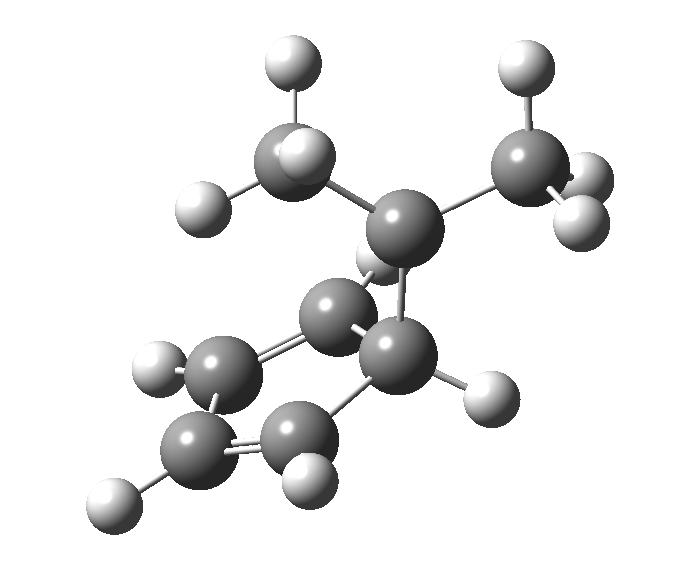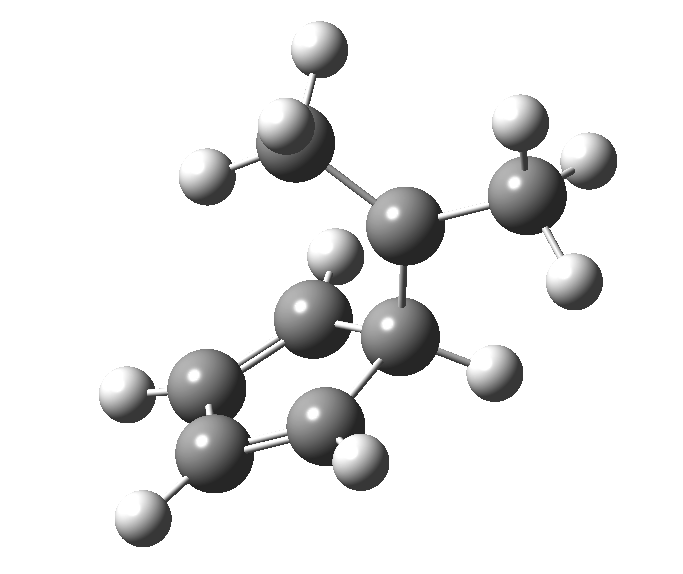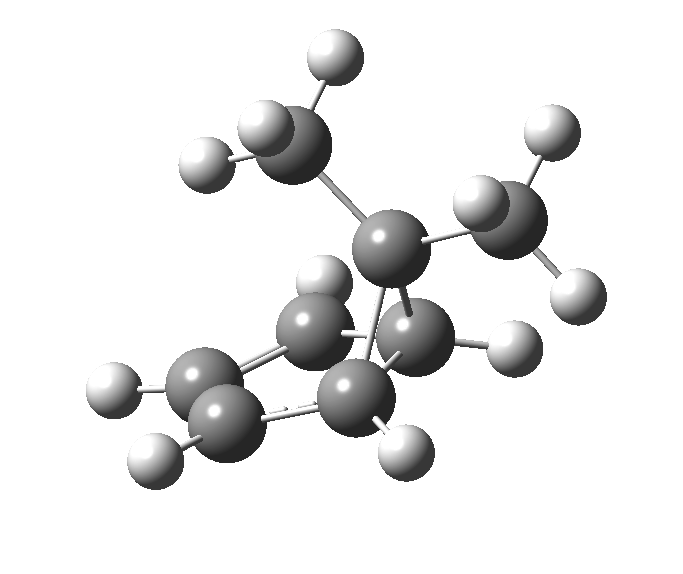 The “forbidden” route corresponds to retention of configuration at the migrating carbon.[2]
The “forbidden” route corresponds to retention of configuration at the migrating carbon.[2] 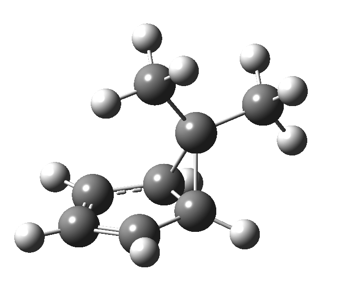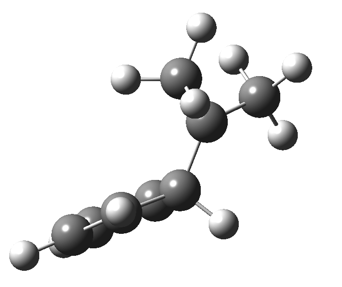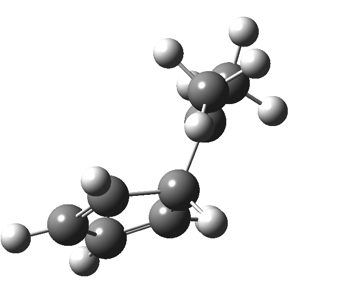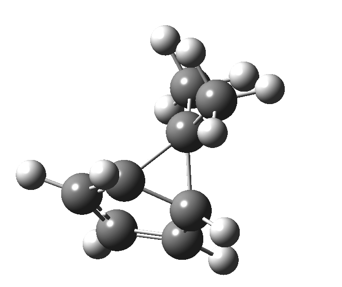 The barriers for each process can be seen below from the IRCs. That for inversion is ~4.5 kcal/mol lower than retention. This nicely transposes the values for the six-electron homologue shown in the previous post.
The barriers for each process can be seen below from the IRCs. That for inversion is ~4.5 kcal/mol lower than retention. This nicely transposes the values for the six-electron homologue shown in the previous post. 
 There is one more nugget of insight that can be extracted. The start/end-point for the six-electron process (homotropylium cation) was, as the name implies, homoaromatic. Now, with a four-electron system we also have an inverse. Nominally, we should now end with homo-antiaromaticity (but see [3]). But antiaromaticity is avoided whenever possible, and so the homoaromatic bond observed in homotropylium is not formed. It resolutely remains a σ-bond (1.48Å) thus sequestering two electrons, and the remaining two electrons simply form a delocalised allyl cation. With the six-electron homotropylium, reactant/product were stabilised by that additional (homo)aromaticity, thus inducing a relatively high barrier. With the four-electron system here, no such reactant/product stabilisation occurs, and hence the reaction barriers are now significantly lower. A rather neat pedagogic example.
There is one more nugget of insight that can be extracted. The start/end-point for the six-electron process (homotropylium cation) was, as the name implies, homoaromatic. Now, with a four-electron system we also have an inverse. Nominally, we should now end with homo-antiaromaticity (but see [3]). But antiaromaticity is avoided whenever possible, and so the homoaromatic bond observed in homotropylium is not formed. It resolutely remains a σ-bond (1.48Å) thus sequestering two electrons, and the remaining two electrons simply form a delocalised allyl cation. With the six-electron homotropylium, reactant/product were stabilised by that additional (homo)aromaticity, thus inducing a relatively high barrier. With the four-electron system here, no such reactant/product stabilisation occurs, and hence the reaction barriers are now significantly lower. A rather neat pedagogic example.
References
- H.S. Rzepa, "Gaussian Job Archive for C8H11(1+)", 2014. https://doi.org/10.6084/m9.figshare.1142175
- H.S. Rzepa, "Gaussian Job Archive for C8H11(1+)", 2014. https://doi.org/10.6084/m9.figshare.1142174
- C.S.M. Allan, and H.S. Rzepa, "Chiral Aromaticities. A Topological Exploration of Möbius Homoaromaticity", Journal of Chemical Theory and Computation, vol. 4, pp. 1841-1848, 2008. https://doi.org/10.1021/ct8001915
Tags: activation energy, reactant/product
Posted in pericyclic, reaction mechanism | 2 Comments »
August 12th, 2014
One thing leads to another. Thus in the previous post, I described a thermal pericyclic reaction that appears to exhibit two transition states resulting in two different stereochemical outcomes. I noted that another such reaction appeared to be a [1,6] carousel migration in homotropylium cation,[1] where transition states for both retention and inversion of the configuration of the migrating group (respectively formally allowed and forbidden) were reported (scheme below). Here I explore this system further.  Firstly, the pathway leading to inversion.[2] The reaction path (ωB97XD/6-311G(d,p)/SCRF=chloroform) has got a very odd (table-top mountain) shape, whereby the region of the transition state (IRC = 0.0) is very flat, and the region close to reactant and (identical) product is very steep. The gradient norm shows this best, with sharp spikes at IRC ± 4.2. Something clearly is happening here to cause this behaviour. Before moving on to analyze this, I want you first to observe the methyl groups below. Note how one of them rotates at the start of the process, and the other at the end. I have elsewhere called this behaviour the methyl flag, and it is due to stereoelectronic re-alignments of the C-H groups accompanying the changes in the conjugated array.
Firstly, the pathway leading to inversion.[2] The reaction path (ωB97XD/6-311G(d,p)/SCRF=chloroform) has got a very odd (table-top mountain) shape, whereby the region of the transition state (IRC = 0.0) is very flat, and the region close to reactant and (identical) product is very steep. The gradient norm shows this best, with sharp spikes at IRC ± 4.2. Something clearly is happening here to cause this behaviour. Before moving on to analyze this, I want you first to observe the methyl groups below. Note how one of them rotates at the start of the process, and the other at the end. I have elsewhere called this behaviour the methyl flag, and it is due to stereoelectronic re-alignments of the C-H groups accompanying the changes in the conjugated array. 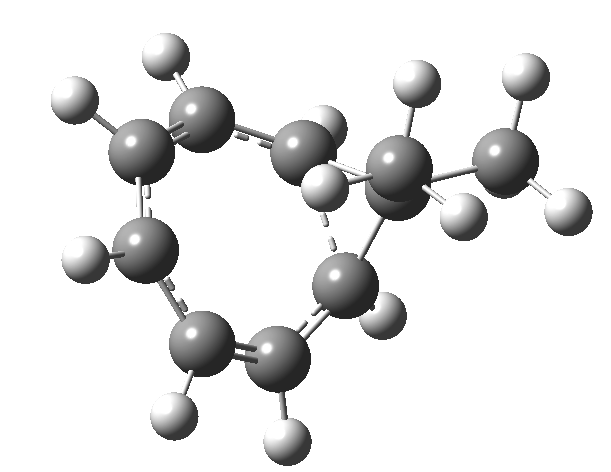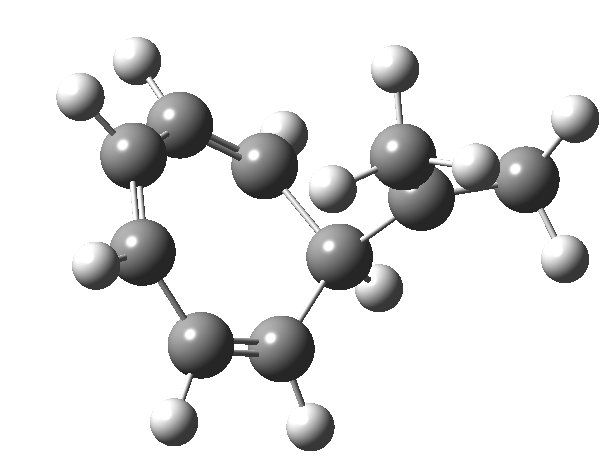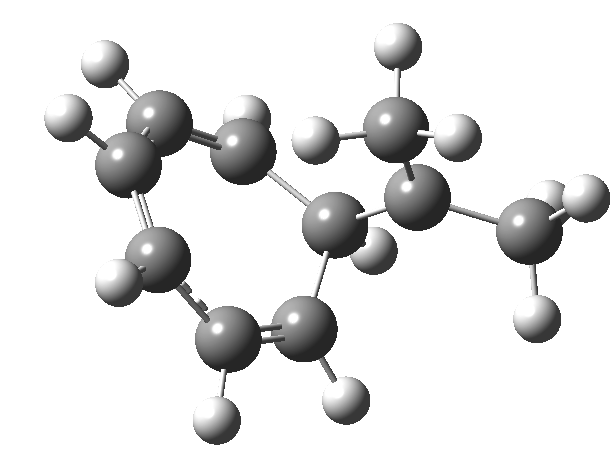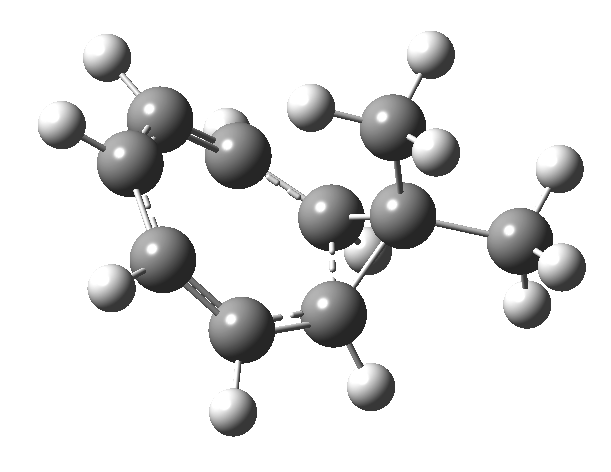

 The homotropylium cation is said to be homoaromatic, indicating that cyclic conjugation can be maintained across a ring in which the σ framework is interrupted at one point. A NICS probe placed at the ring critical point of this molecule reveals a chemical shift of -11.3 ppm[3], very similar to eg that obtained for benzene itself. The three highest doubly occupied NBOs (below) show two normal π-type orbitals and one rather different one that spans the homo-bond (the MOs, before you ask, are a bit of a mess, with lots of mixed contributions from other parts of the σ framework).
The homotropylium cation is said to be homoaromatic, indicating that cyclic conjugation can be maintained across a ring in which the σ framework is interrupted at one point. A NICS probe placed at the ring critical point of this molecule reveals a chemical shift of -11.3 ppm[3], very similar to eg that obtained for benzene itself. The three highest doubly occupied NBOs (below) show two normal π-type orbitals and one rather different one that spans the homo-bond (the MOs, before you ask, are a bit of a mess, with lots of mixed contributions from other parts of the σ framework).
Read the rest of this entry »
References
- A.M. Genaev, G.E. Sal’nikov, and V.G. Shubin, "Energy barriers to carousel rearrangements of carbocations: Quantum-chemical calculations vs. experiment", Russian Journal of Organic Chemistry, vol. 43, pp. 1134-1138, 2007. https://doi.org/10.1134/s1070428007080076
- H.S. Rzepa, "Gaussian Job Archive for C10H13(1+)", 2014. https://doi.org/10.6084/m9.figshare.1134556
- H.S. Rzepa, "Gaussian Job Archive for C10H13(1+)", 2014. https://doi.org/10.6084/m9.figshare.1135694
Tags: chemical shift, higher energy, Sangean Table Top Portable Audio Device
Posted in pericyclic, reaction mechanism | 5 Comments »
August 10th, 2014
In my first post on the topic, I discussed how inverting the polarity of the C-X bond from X=O to X=Be (scheme below) could flip the stereochemical course of the electrocyclic pericyclic reaction of a divinyl system. This was followed up by exploring what happens at the half way stage, i.e. X=CH2, the answer being that one gets an antarafacial pathway as with X=O. Here I fill in another gap, X=BH to see if a metaphorical microscope can be used to view the actual region of the “flip” to a suprafacial mode. This time, uniquely, it proved possible to locate TWO transition states for this process, one suprafacial[1] and one antarafacial[2], this latter being 10.5 kcal/mol lower in ΔG† (ωB97XD/6-311G(d,p)/SCRF=dichloromethane). It is quite rare to be able to find BOTH stereochemical outcomes of a thermal pericyclic reaction.‡
This time, uniquely, it proved possible to locate TWO transition states for this process, one suprafacial[1] and one antarafacial[2], this latter being 10.5 kcal/mol lower in ΔG† (ωB97XD/6-311G(d,p)/SCRF=dichloromethane). It is quite rare to be able to find BOTH stereochemical outcomes of a thermal pericyclic reaction.‡
Read the rest of this entry »
References
- H.S. Rzepa, "Gaussian Job Archive for C5H7B", 2014. https://doi.org/10.6084/m9.figshare.1133933
- H.S. Rzepa, "Gaussian Job Archive for C5H7B", 2014. https://doi.org/10.6084/m9.figshare.1133934
Tags: Alex Genaev, Hawaii, HB
Posted in pericyclic, reaction mechanism | No Comments »
August 6th, 2014
In my earlier post on the topic, I discussed how inverting the polarity of the C-X bond from X=O to X=Be could flip the stereochemical course of the electrocyclic pericyclic reaction of a divinyl system. An obvious question would be: what happens at the half way stage, ie X=CH2? Well, here is the answer. 
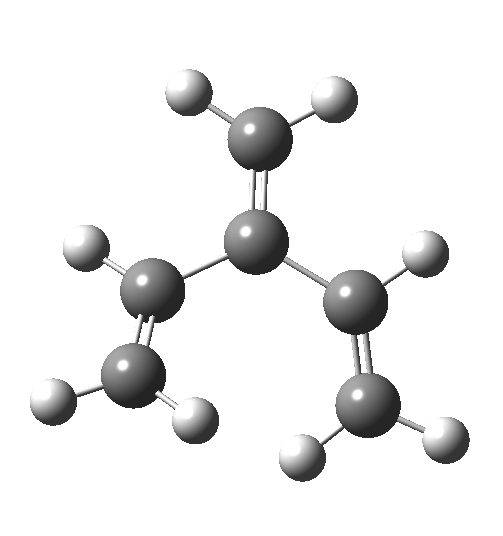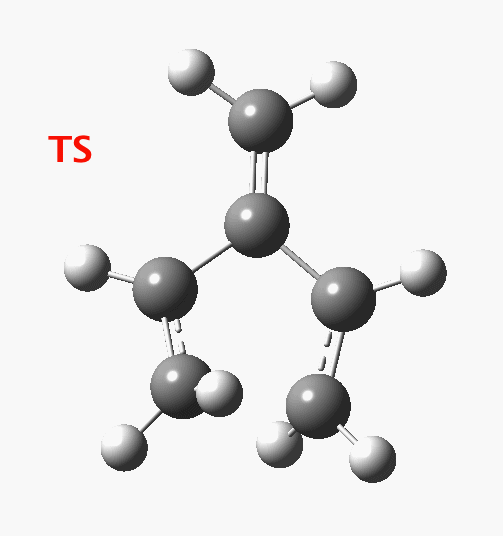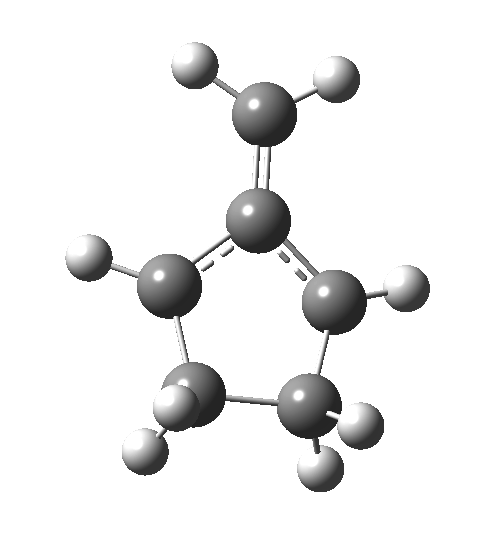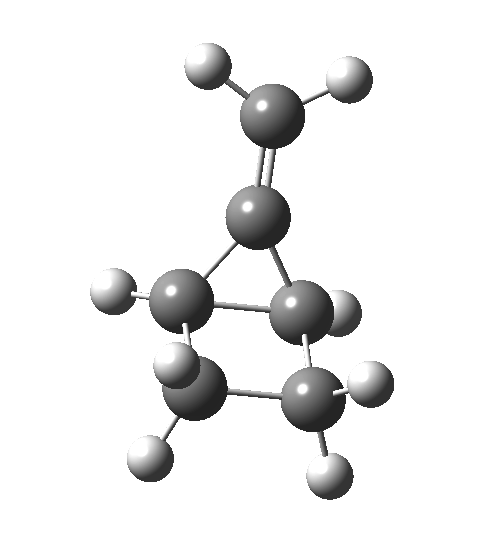 The reaction occurs in two stages (ωB97XD/6-311G(d,p)/SCRF=dichloromethane)[1] but overall is a concerted, albeit asynchronous, reaction. The initial stage is a conrotatory ring closure (as observed with X=O but opposite to X=Be), and reaching what we will call a HI (hidden intermediate). This HI clearly has zwitterionic character, and manifests its presence most obviously at IRC = -3.5 below.
The reaction occurs in two stages (ωB97XD/6-311G(d,p)/SCRF=dichloromethane)[1] but overall is a concerted, albeit asynchronous, reaction. The initial stage is a conrotatory ring closure (as observed with X=O but opposite to X=Be), and reaching what we will call a HI (hidden intermediate). This HI clearly has zwitterionic character, and manifests its presence most obviously at IRC = -3.5 below. 
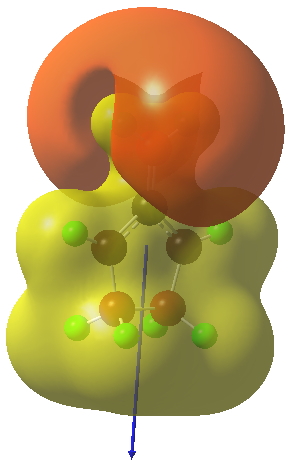
 The polarity of this HI is revealed by the dipole moment (6D) and molecular electrostatic potentials, below. The dipole vector goes from -ve to +ve, and the MEP clearly reveals the polarity below.
The polarity of this HI is revealed by the dipole moment (6D) and molecular electrostatic potentials, below. The dipole vector goes from -ve to +ve, and the MEP clearly reveals the polarity below. 
 This ionic HI however is not stable, and in the second stage of the reaction collapses to the neutral bicyclic hydrocarbon shown below. Overall, it amounts to a 2+2 cycloaddition, but with a very unusual pathway in which one C-C bond is very much formed before the other (which is how the reaction escapes the clutches of the Woodward-Hoffmann forbidden-ness).
This ionic HI however is not stable, and in the second stage of the reaction collapses to the neutral bicyclic hydrocarbon shown below. Overall, it amounts to a 2+2 cycloaddition, but with a very unusual pathway in which one C-C bond is very much formed before the other (which is how the reaction escapes the clutches of the Woodward-Hoffmann forbidden-ness).  Why is all this worth this follow-up? Well, one can now start to “design” the reaction. All three carbon atoms with formal charges can be stabilised or destabilised with appropriate substituents. It should not be too difficult to stabilise out the HI into just an I(intermediate), or indeed to remove it from the profile. Nice perhaps for a group of students, who can partition up the substituents amongst themselves and discover if they have the desired effect. And would any of this tinkering change the stereochemical outcome?
Why is all this worth this follow-up? Well, one can now start to “design” the reaction. All three carbon atoms with formal charges can be stabilised or destabilised with appropriate substituents. It should not be too difficult to stabilise out the HI into just an I(intermediate), or indeed to remove it from the profile. Nice perhaps for a group of students, who can partition up the substituents amongst themselves and discover if they have the desired effect. And would any of this tinkering change the stereochemical outcome?
References
- H.S. Rzepa, "Gaussian Job Archive for C6H8", 2014. https://doi.org/10.6084/m9.figshare.1128205
Tags: Hawaii
Posted in pericyclic | No Comments »
August 6th, 2014
I do go on a lot about the importance of having modern access to data. And so the appearance of this article[1] immediately struck me as important. It is appropriately enough in the new journal Scientific Data. The data contain computed properties at the B3LYP/6-31G(2df,p) level for 133,885 species with up to nine heavy atoms, and the entire data set has its own DOI[2]. The data is generated by subjecting a molecule set to a number of validation protocols, including obtaining relaxed (optimised) geometries at the B3LYP/6-31G(2df,p) level. It would be good to replicate this set with inclusion of a functional that also includes dispersion, and of course making the coordinates all available in this manner greatly facilitates this. The collection also includes data for e.g. 6095 constitutional isomers of C7H10O2, which reminds me of an early, delightfully entitled, article adopting such an approach in quantum chemistry[3]. Such collections are an important part of the process of validating computational methods[4] This way of publishing data does raise some interesting discussion points.
Read the rest of this entry »
References
- R. Ramakrishnan, P.O. Dral, M. Rupp, and O.A. von Lilienfeld, "Quantum chemistry structures and properties of 134 kilo molecules", Scientific Data, vol. 1, 2014. https://doi.org/10.1038/sdata.2014.22
- Raghunathan Ramakrishnan., P. Dral, P.O. Dral, M. Rupp, and O. Anatole Von Lilienfeld., "Quantum chemistry structures and properties of 134 kilo molecules", 2014. https://doi.org/10.6084/m9.figshare.978904
- P.P. Bera, K.W. Sattelmeyer, M. Saunders, H.F. Schaefer, and P.V.R. Schleyer, "Mindless Chemistry", The Journal of Physical Chemistry A, vol. 110, pp. 4287-4290, 2006. https://doi.org/10.1021/jp057107z
- P. Murray-Rust, H.S. Rzepa, J.J.P. Stewart, and Y. Zhang, "A global resource for computational chemistry", Journal of Molecular Modeling, vol. 11, pp. 532-541, 2005. https://doi.org/10.1007/s00894-005-0278-1
Tags: Much software, validation protocols
Posted in Chemical IT | 5 Comments »
August 4th, 2014
The outcome of pericyclic reactions con depend most simply on three conditions, any two of which determine the third. Whether the catalyst is Δ or hν (heat or light), the topology determining any stereochemistry and the participating electron count (4n+2/4n). It is always neat to conjure up a simple switch to toggle these; heat or light is simple, but what are the options for toggling the electron count? Here is one I have contrived by playing a game with the periodic table.  The ring closure of a divinylketone is called the Nazarov reaction, it being promoted thermodynamically by coordination of a Lewis acid to atom X. Divinyl ketone can be regarded as a hidden pentadienyl cation, since the C=O bond is polarised Cδ+Oδ- in the time-honoured manner of organic chemistry. In this (formal) resonance form, it becomes part of a pentadienyl cation and can electrocyclise via a 4-electron reaction involving a stereochemical process known as conrotation. The new bond is formed antarafacially (from opposite faces) at the termini of the pentadienyl cation (ωB97XD/6-311G(d,p)/SCRF=dichloromethane.[1]). Note that for the uncatalysed reaction, the barrier is high and the reaction is endothermic but adding a BF3 to the oxygen lowers the barrier and removes the endothermicity.[2]
The ring closure of a divinylketone is called the Nazarov reaction, it being promoted thermodynamically by coordination of a Lewis acid to atom X. Divinyl ketone can be regarded as a hidden pentadienyl cation, since the C=O bond is polarised Cδ+Oδ- in the time-honoured manner of organic chemistry. In this (formal) resonance form, it becomes part of a pentadienyl cation and can electrocyclise via a 4-electron reaction involving a stereochemical process known as conrotation. The new bond is formed antarafacially (from opposite faces) at the termini of the pentadienyl cation (ωB97XD/6-311G(d,p)/SCRF=dichloromethane.[1]). Note that for the uncatalysed reaction, the barrier is high and the reaction is endothermic but adding a BF3 to the oxygen lowers the barrier and removes the endothermicity.[2] 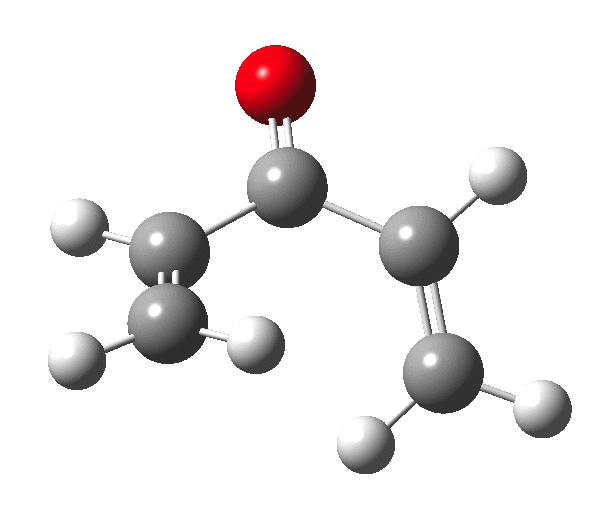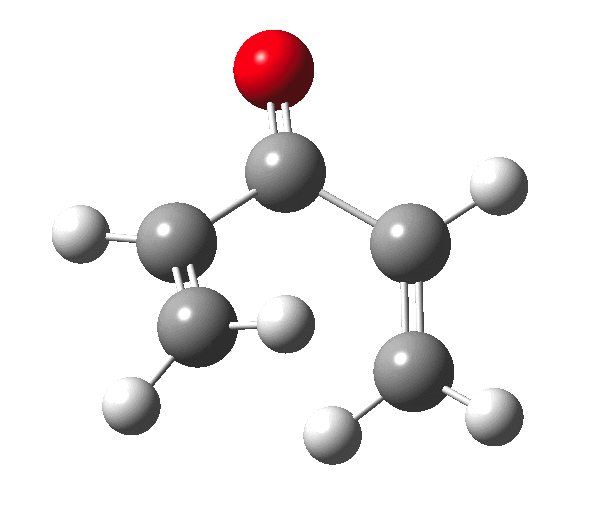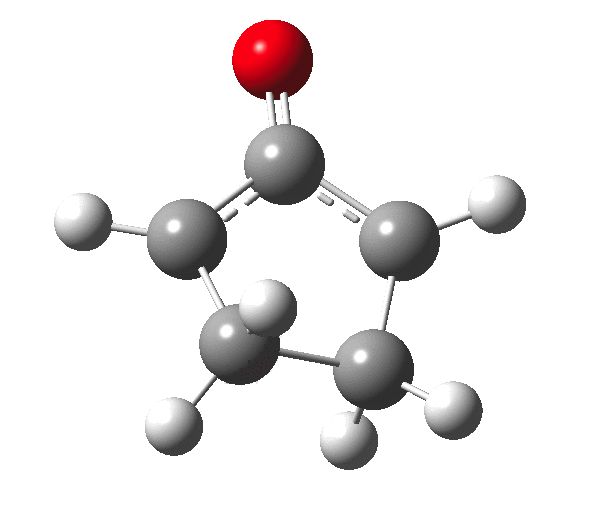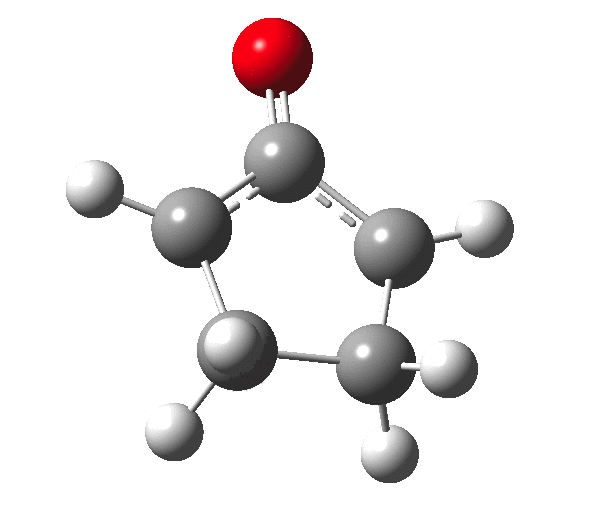

 So, one can play a game and ask what would happen if the polarity of the C=X bond were to be reversed. This means going left of oxygen in the periodic table, ending at Be.[3] The reaction has a high barrier, but it is strongly exothermic.† However the most noteworthy aspect is that the stereochemistry of the electrocyclisation is now disrotatory, with suprafacial bond formation (from the bottom face in the animation below). The stereochemical outcome of this reaction has been flipped by reversing the polarity of the CX bond.‡
So, one can play a game and ask what would happen if the polarity of the C=X bond were to be reversed. This means going left of oxygen in the periodic table, ending at Be.[3] The reaction has a high barrier, but it is strongly exothermic.† However the most noteworthy aspect is that the stereochemistry of the electrocyclisation is now disrotatory, with suprafacial bond formation (from the bottom face in the animation below). The stereochemical outcome of this reaction has been flipped by reversing the polarity of the CX bond.‡ 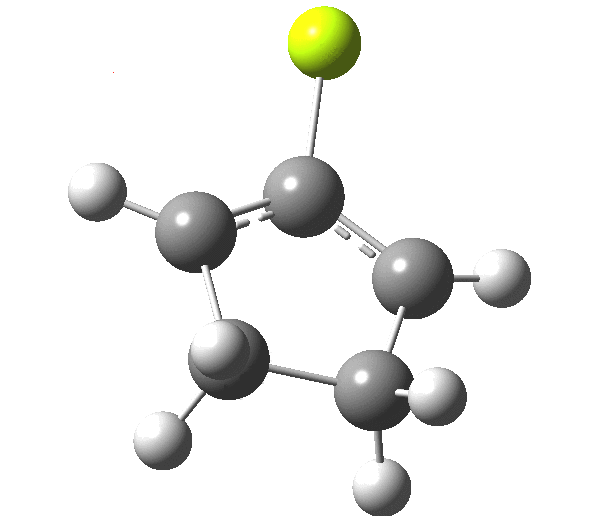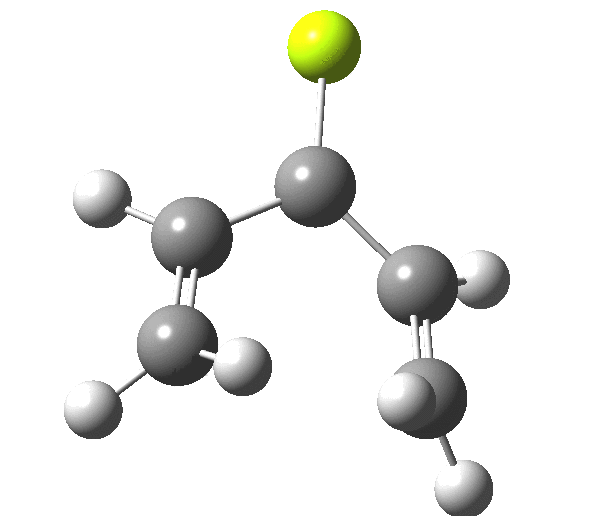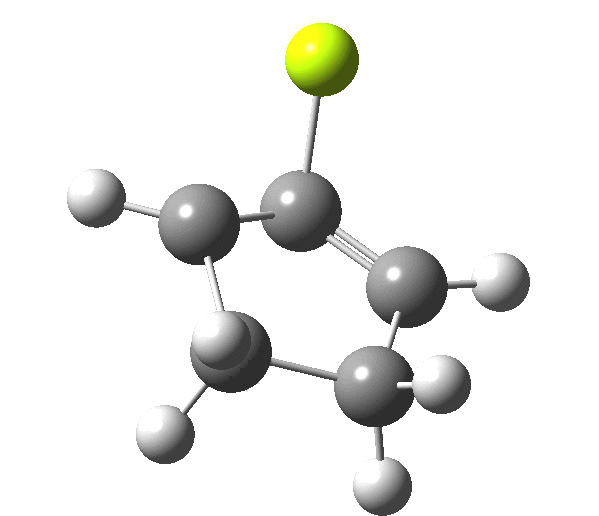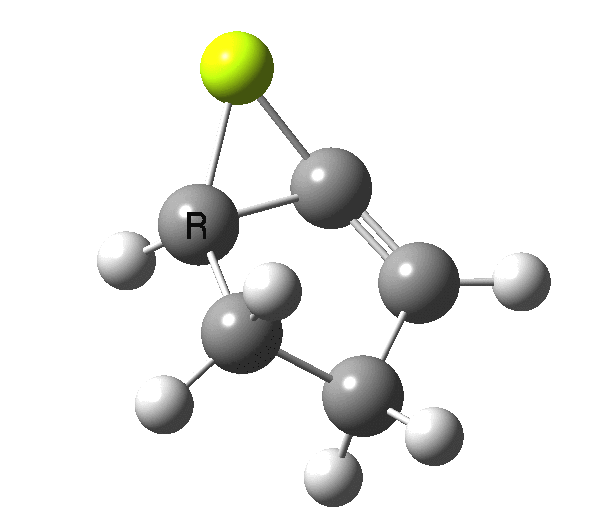
 This little example shows how a thought game played using the periodic table can then be reality tested by solving appropriate quantum mechanical equations. In this instance, one is not going to rush into the laboratory to try to replicate the experiment, but it might help catalyse new thoughts amongst the readers of this blog.
This little example shows how a thought game played using the periodic table can then be reality tested by solving appropriate quantum mechanical equations. In this instance, one is not going to rush into the laboratory to try to replicate the experiment, but it might help catalyse new thoughts amongst the readers of this blog.
Read the rest of this entry »
References
- H.S. Rzepa, "Gaussian Job Archive for C5H6O", 2014. https://doi.org/10.6084/m9.figshare.1125721
- H.S. Rzepa, "Gaussian Job Archive for C5H6BF3O", 2014. https://doi.org/10.6084/m9.figshare.1125724
- H.S. Rzepa, "Gaussian Job Archive for C5H6Be", 2014. https://doi.org/10.6084/m9.figshare.1125792
Tags: animation
Posted in Interesting chemistry, pericyclic | No Comments »
July 19th, 2014
Whilst clusters of carbon atoms are well-known, my eye was caught by a recent article describing the detection of a cluster of boron atoms, B40 to be specific.[1] My interest was in how the σ and π-electrons were partitioned. In a C40, one can reliably predict that each carbon would contribute precisely one π-electron. But boron, being more electropositive, does not always play like that. Having one electron less per atom, one might imagine that a fullerene-like boron cluster would have no π-electrons. But the element has a propensity[2] to promote its σ-electrons into the π-manifold, leaving a σ-hole. So how many π-electrons does B40 have? These sorts of clusters are difficult to build using regular structure editors, and so coordinates are essential. The starting point for a set of coordinates with which to compute a wavefunction was the supporting information. Here is the relevant page: 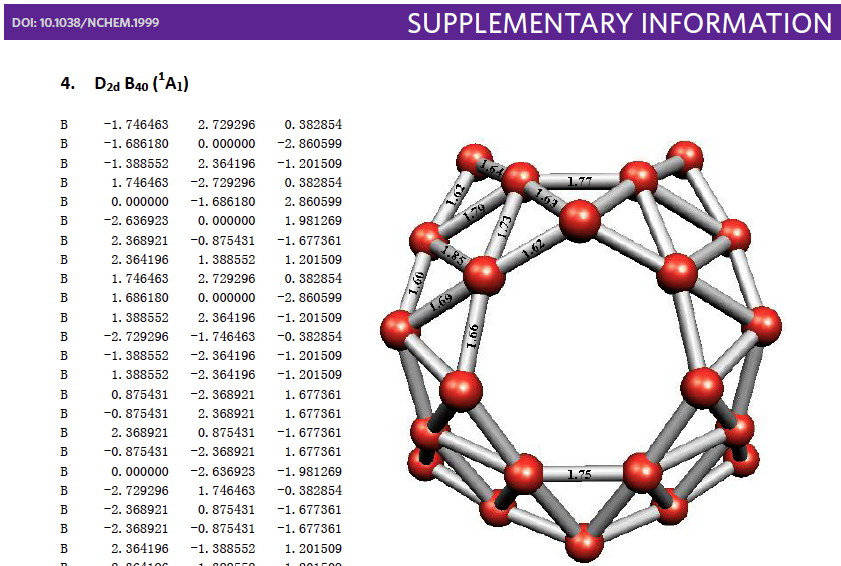 The coordinates are certainly there (that is not always the case), but you have to know a few tricks to make them usable.
The coordinates are certainly there (that is not always the case), but you have to know a few tricks to make them usable.
Read the rest of this entry »
References
- H. Zhai, Y. Zhao, W. Li, Q. Chen, H. Bai, H. Hu, Z.A. Piazza, W. Tian, H. Lu, Y. Wu, Y. Mu, G. Wei, Z. Liu, J. Li, S. Li, and L. Wang, "Observation of an all-boron fullerene", Nature Chemistry, vol. 6, pp. 727-731, 2014. https://doi.org/10.1038/nchem.1999
- H.S. Rzepa, "The distortivity of π-electrons in conjugated boron rings", Physical Chemistry Chemical Physics, vol. 11, pp. 10042, 2009. https://doi.org/10.1039/b911817a
Tags: Acrobat, Adobe, chemical markup, DOS, operating systems, PDF, pence, Unix
Posted in Chemical IT, Interesting chemistry | 2 Comments »
July 12th, 2014
Computational quantum chemistry has made fantastic strides in the last 30 years. Often deep insight into all sorts of questions regarding reactions and structures of molecules has become possible. But sometimes the simplest of questions can prove incredibly difficult to answer. One such is how accurately can the boiling point of water be predicted from first principles? Or its melting point? Another classic case is why mercury is a liquid at room temperatures? The answer to that question (along with another, why is gold the colour it is?) is often anecdotally attributed to Einstein. More accurately, to his special theory of relativity.[1] But finally in 2013 a computational proof of this was demonstrated for mercury.[2] The proof was built up in three stages.
Read the rest of this entry »
References
- A. Einstein, "Ist die Trägheit eines Körpers von seinem Energieinhalt abhängig?", Annalen der Physik, vol. 323, pp. 639-641, 1905. https://doi.org/10.1002/andp.19053231314
- F. Calvo, E. Pahl, M. Wormit, and P. Schwerdtfeger, "Evidence for Low‐Temperature Melting of Mercury owing to Relativity", Angewandte Chemie International Edition, vol. 52, pp. 7583-7585, 2013. https://doi.org/10.1002/anie.201302742
Tags: bulk metal, pence, potential energy surfaces
Posted in General | 4 Comments »
July 5th, 2014
I was lucky enough to attend the announcement made in 2012 of the discovery of the Higgs Boson. It consisted of a hour-long talk mostly about statistics, and how the particle physics community can only claim a discovery when their data has achieved a 5σ confidence level. This represents a 1 in 3.5 million probability of the result occurring by chance. I started thinking: how much chemistry is asserted at that level of confidence? Today, I read Steve Bachrach’s post on the structure of Citrinalin B and how “use of Goodman’s DP4 method indicates a 100% probability that the structure of citrinalin B is (the structure below)”. Wow, that is even higher than the physicists. Of course, 100% has been obtained by rounding 99.7 (3σ is 99.73%) or whatever (this is one number that should never be so rounded!). 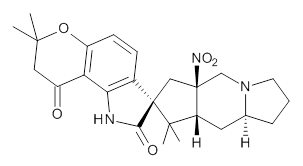 But there was one aspect of this that I did want to have a confidence level for; the absolute configuration of citrinalin B. Reading the article Steve quotes[1], one sees this aspect is attributed to ref 5[2], dating from 2005. There the configuration was assigned on the basis of “comparison of the electronic circular dichroism (ECD) spectra for 1 and 2 with those of known spirooxiindole alkaloids“. However, this method can fail[3]. Also, one finds “comparison of the vibrational circular dichroism (VCD) spectra of 1 with those of model compounds“[2]. Nowadays, one would say that there is no need for model compounds, why not measure and compute the VCD of the actual compound? Even a determination using the Flack crystallographic method can occasionally be wrong![4]. Which leads to asking what typical confidence levels might be for these three techniques, and indeed whether improving instrumentation means that the confidence level gets higher with time. OK, I am going to guess these.
But there was one aspect of this that I did want to have a confidence level for; the absolute configuration of citrinalin B. Reading the article Steve quotes[1], one sees this aspect is attributed to ref 5[2], dating from 2005. There the configuration was assigned on the basis of “comparison of the electronic circular dichroism (ECD) spectra for 1 and 2 with those of known spirooxiindole alkaloids“. However, this method can fail[3]. Also, one finds “comparison of the vibrational circular dichroism (VCD) spectra of 1 with those of model compounds“[2]. Nowadays, one would say that there is no need for model compounds, why not measure and compute the VCD of the actual compound? Even a determination using the Flack crystallographic method can occasionally be wrong![4]. Which leads to asking what typical confidence levels might be for these three techniques, and indeed whether improving instrumentation means that the confidence level gets higher with time. OK, I am going to guess these.
Read the rest of this entry »
References
- E.V. Mercado-Marin, P. Garcia-Reynaga, S. Romminger, E.F. Pimenta, D.K. Romney, M.W. Lodewyk, D.E. Williams, R.J. Andersen, S.J. Miller, D.J. Tantillo, R.G.S. Berlinck, and R. Sarpong, "Total synthesis and isolation of citrinalin and cyclopiamine congeners", Nature, vol. 509, pp. 318-324, 2014. https://doi.org/10.1038/nature13273
- T. Mugishima, M. Tsuda, Y. Kasai, H. Ishiyama, E. Fukushi, J. Kawabata, M. Watanabe, K. Akao, and J. Kobayashi, "Absolute Stereochemistry of Citrinadins A and B from Marine-Derived Fungus", The Journal of Organic Chemistry, vol. 70, pp. 9430-9435, 2005. https://doi.org/10.1021/jo051499o
- F. Cherblanc, Y. Lo, E. De Gussem, L. Alcazar‐Fuoli, E. Bignell, Y. He, N. Chapman‐Rothe, P. Bultinck, W.A. Herrebout, R. Brown, H.S. Rzepa, and M.J. Fuchter, "On the Determination of the Stereochemistry of Semisynthetic Natural Product Analogues using Chiroptical Spectroscopy: Desulfurization of Epidithiodioxopiperazine Fungal Metabolites", Chemistry – A European Journal, vol. 17, pp. 11868-11875, 2011. https://doi.org/10.1002/chem.201101129
- F.L. Cherblanc, Y. Lo, W.A. Herrebout, P. Bultinck, H.S. Rzepa, and M.J. Fuchter, "Mechanistic and Chiroptical Studies on the Desulfurization of Epidithiodioxopiperazines Reveal Universal Retention of Configuration at the Bridgehead Carbon Atoms", The Journal of Organic Chemistry, vol. 78, pp. 11646-11655, 2013. https://doi.org/10.1021/jo401316a
Tags: chemical, Reading, Steve Bachrach, X-ray
Posted in General | 2 Comments »
NMR evidence clearly pointed to the π-complex A as being formed, and not the cyclic nitrosonium species B (X=Al4–). If you are wondering where you have seen an analogy for the latter, it would be the species formed when bromine reacts with an alkene (≡ Br+, X=Br– or Br3–). The two structures are shown below[1]
 Since the topic that sparked this concerned pericyclic reactions, it seemed possible that if it had been formed, species B would immediately undergo a pericyclic electrocyclic reaction to form the rather odd-looking cation C, which might then be trapped by eg X(-) to form the nitrone D. So this post is an exploration of what happens when X-NO (X= CF3COO, trifluoracetate) interacts with 2,3-dimethyl-2-butene, as an illustration of what can be achieved nowadays from about 2 days worth of dry-lab computation as a prelude to e.g. an experiment in the wet-lab (it would take a little more than two days to achieve the latter I suspect). Hence computationally directed synthesis. The model is set up as ωB97XD/6-311G(d,p)/SCRF=chloroform. A transition state is located[2] and the resulting IRC (below) [3] does not quite have the outcome the above scheme would suggest.
Since the topic that sparked this concerned pericyclic reactions, it seemed possible that if it had been formed, species B would immediately undergo a pericyclic electrocyclic reaction to form the rather odd-looking cation C, which might then be trapped by eg X(-) to form the nitrone D. So this post is an exploration of what happens when X-NO (X= CF3COO, trifluoracetate) interacts with 2,3-dimethyl-2-butene, as an illustration of what can be achieved nowadays from about 2 days worth of dry-lab computation as a prelude to e.g. an experiment in the wet-lab (it would take a little more than two days to achieve the latter I suspect). Hence computationally directed synthesis. The model is set up as ωB97XD/6-311G(d,p)/SCRF=chloroform. A transition state is located[2] and the resulting IRC (below) [3] does not quite have the outcome the above scheme would suggest. 
Neither A nor B is formed; instead it is the tetrahedral species E, which is ~15 kcal/mol endothermic.
I should immediately point out that this is not inconsistent with the formation of A as previously characterised[1]. That is because this experiment was conducted with a non-nucleophilic counter-anion (X=Al4–), whereas in the computational simulation above, we have a nucleophilic anion (X= CF3CO2–). What a difference the inclusion of a counter-ion in the calculation can have! The barrier however (~35 kcal/mol) is a little too high for a facile thermal reaction. In the second of this two-stage reaction, E now ring-opens to form the anticipated D[4] with quite a small barrier of ~6 kcal/mol, but a highly exothermic outcome. I ask this question about it; can this still be described as a pericyclic process? (there is some analogy to the electrocyclic ring opening of a cyclopropyl tosylate).
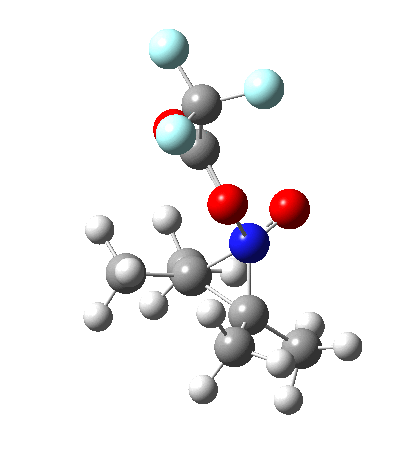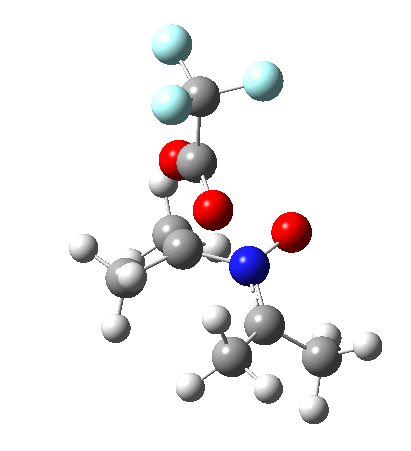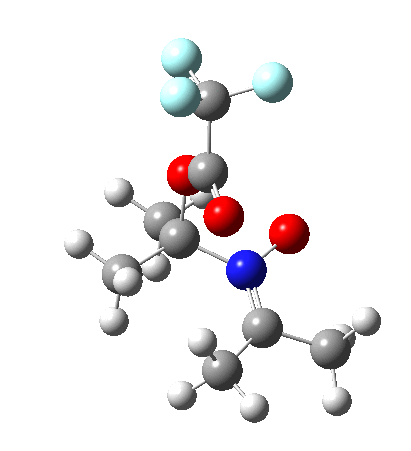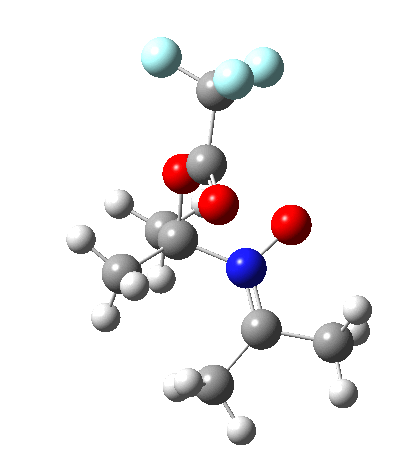
So what are the conclusions? Well, because of the rather high initial barrier, the alkene will need activation (by electron donating substituents, perhaps OMe) for the reaction to become more viable. But if it works, it could be an interesting synthesis of nitrones (I have not yet searched to find out if the reaction is actually known).








 But there was one aspect of this that I did want to have a confidence level for; the absolute configuration of citrinalin B. Reading the article Steve quotes
But there was one aspect of this that I did want to have a confidence level for; the absolute configuration of citrinalin B. Reading the article Steve quotes