July 3rd, 2014
Increasingly, our access to scientific information is becoming a research topic in itself. Thus an analysis of big deal journal bundles[1] has attracted much interesting commentary (including one from a large scientific publisher[2]). In the UK, our funding councils have been pro-active in promoting the so-called GOLD publishing model, where the authors (aided by grants from their own institution or others) pay the perpetual up-front publication costs (more precisely the costs demanded by the publishers, which is not necessarily the same thing) so that their article is removed from the normal subscription pay wall erected by the publisher and becomes accessible to anyone. As the proportion of GOLD content increases, it was anticipated (hoped?) that the costs of accessing the remaining non-GOLD articles via a pay-walled subscription would decrease.
Read the rest of this entry »
References
- T.C. Bergstrom, P.N. Courant, R.P. McAfee, and M.A. Williams, "Evaluating big deal journal bundles", Proceedings of the National Academy of Sciences, vol. 111, pp. 9425-9430, 2014. https://doi.org/10.1073/pnas.1403006111
- C. Woolston, "Secret publishing deals exposed", Nature, vol. 510, pp. 447-447, 2014. https://doi.org/10.1038/510447f
Tags: GBP, typical article processing charge ranges, United Kingdom
Posted in Chemical IT, General | 8 Comments »
June 26th, 2014
The Bürgi–Dunitz angle describes the trajectory of an approaching nucleophile towards the carbon atom of a carbonyl group. A colleague recently came to my office to ask about the inverse, that is what angle would an electrophile approach (an amide)? Thus it might approach either syn or anti with respect to the nitrogen, which is a feature not found with nucleophilic attack.  My first thought was to calculate the wavefunction and identify the location and energy (= electrophilicity) of the lone pairs (the presumed attractor of an electrophile). But a better more direct approach soon dawned. A search of the crystal structure database. Here is the search definition, with the C=O-E angle, the O-E distance and the N-C=O-E torsion defined (also specified for R factor < 5%, no errors and no disorder).
My first thought was to calculate the wavefunction and identify the location and energy (= electrophilicity) of the lone pairs (the presumed attractor of an electrophile). But a better more direct approach soon dawned. A search of the crystal structure database. Here is the search definition, with the C=O-E angle, the O-E distance and the N-C=O-E torsion defined (also specified for R factor < 5%, no errors and no disorder). 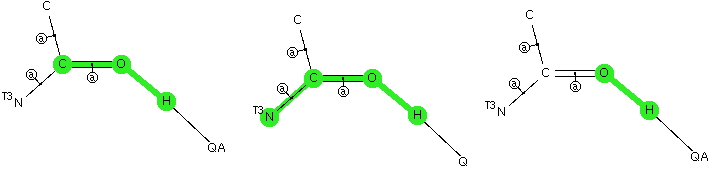 The first plot is of the torsion vs the distance, for E = H-X (X=O,F, Cl)
The first plot is of the torsion vs the distance, for E = H-X (X=O,F, Cl) 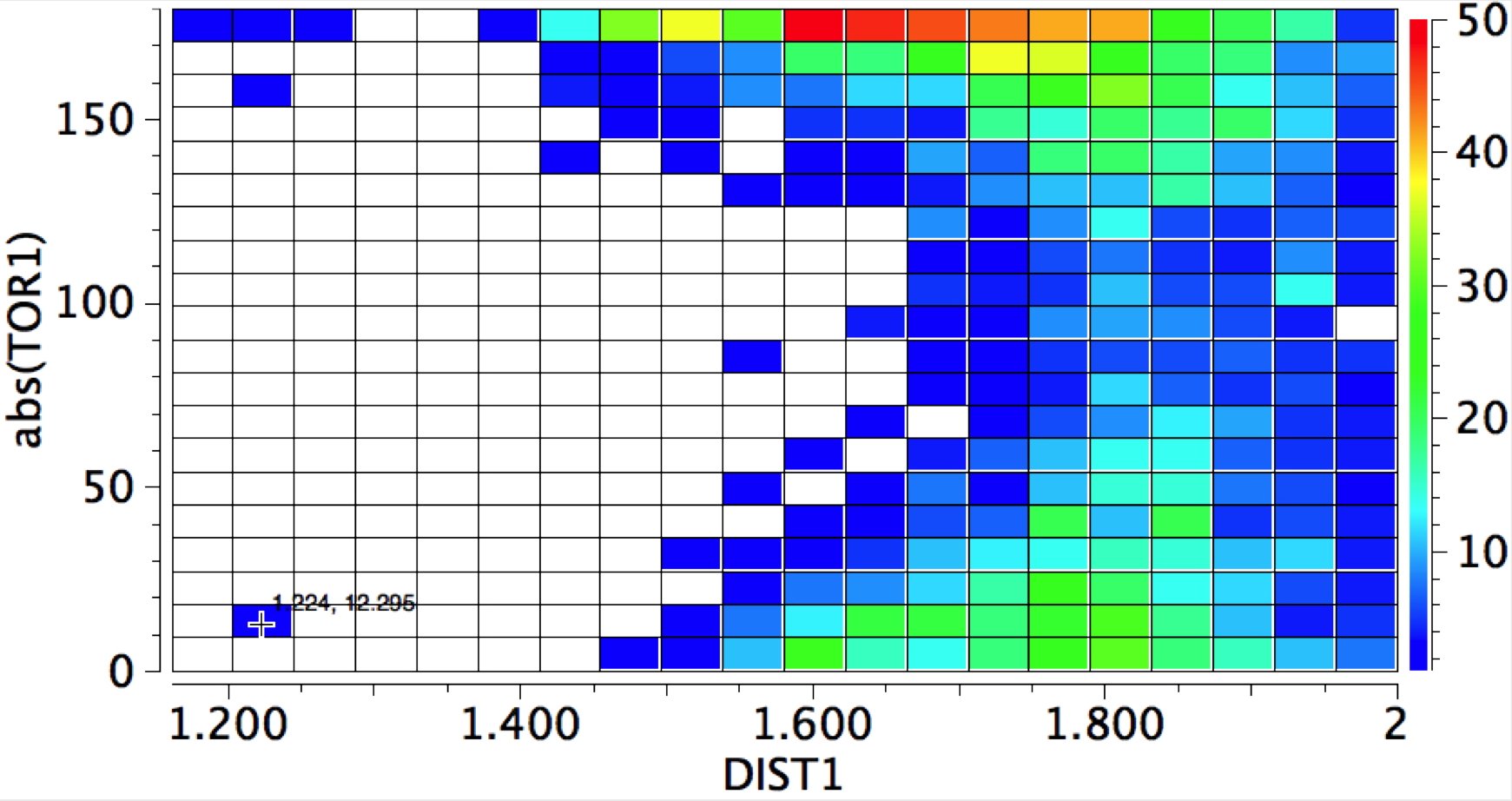
Read the rest of this entry »
Tags: electronics, energy, metal results, metal-π-bond interactions, search definition
Posted in crystal_structure_mining, General, Interesting chemistry | No Comments »
June 18th, 2014
I was reminded of this article by Michelle Francl[1], where she poses the question “What anchor values would most benefit students as they seek to hone their chemical intuition?” She gives as common examples: room temperature is 298.17K (actually 300K, but perhaps her climate is warmer than that of the UK!), the length of a carbon-carbon single bond, the atomic masses of the more common elements.
Read the rest of this entry »
References
- M. Francl, "Take a number", Nature Chemistry, vol. 5, pp. 725-726, 2013. https://doi.org/10.1038/nchem.1733
Tags: /RT, chemical intuition, chemical timescale, chemical timescales, favourite chemical anchor, Michelle Francl, possible chemical reaction, United Kingdom
Posted in General | 2 Comments »
June 8th, 2014
A word of explanation about this test page for experimenting with JSmol. Many moons ago I posted about how to include a generated 3D molecular model in a blog post, and have used that method on many posts here ever since. It relied on Java as the underlying software (first introduced in 1996), or almost 20 years ago. Like most software technologies, much has changed, and Java itself (as a compiled language) has had to move to improve its underlying security. In the last year, the Java code itself (in this case Jmol) has needed to be digitally signed in a standard manner, and this meant that many an old site that used unsigned older versions has started to throw up increasingly alarming messages.
Read the rest of this entry »
Tags: Bob Hanson, Java, JavaScript, Jim Hu, software technologies, Takanori Nakane, wonderful technology
Posted in Chemical IT | 1 Comment »
June 6th, 2014
Following the discussion here of Kekulé’s suggestion of what we now call a vibrational mode (and which in fact now bears his name), I thought I might apply the concept to a recent molecule known as [2.2]paracyclophane. The idea was sparked by Steve Bachrach’s latest post, where the “zero-point” structure of the molecule has recently been clarified as having D2 symmetry.[1]
Read the rest of this entry »
References
- H. Wolf, D. Leusser, M. Jørgensen, R. Herbst‐Irmer, Y. Chen, E. Scheidt, W. Scherer, B.B. Iversen, and D. Stalke, "Phase Transition of [2,2]‐Paracyclophane – An End to an Apparently Endless Story", Chemistry – A European Journal, vol. 20, pp. 7048-7053, 2014. https://doi.org/10.1002/chem.201304972
Tags: 10.1002, 201304972, Hiberty and co, Steve Bachrach
Posted in Historical, Interesting chemistry | No Comments »
May 28th, 2014
In the preceding post, a nice discussion broke out about Kekulé’s 1872 model for benzene.[1] This model has become known as the oscillation hypothesis between two extreme forms of benzene (below). The discussion centered around the semantics of the term oscillation compared to vibration (a synonym or not?) and the timescale implied by each word. The original article is in german, but more significantly, obtainable only with difficulty. Thus I cannot access[1] the article directly since my university does not have the appropriate “back-number” subscription.‡ So it was with delight that I tracked down an English translation in a journal that I could easily access.[2] Here I discuss what I found (on pages 614-615, the translation does not have its own DOI).
Read the rest of this entry »
References
- A. Kekulé, "Ueber einige Condensationsproducte des Aldehyds", Justus Liebigs Annalen der Chemie, vol. 162, pp. 77-124, 1872. https://doi.org/10.1002/jlac.18721620110
- "Organic chemistry", Journal of the Chemical Society, vol. 25, pp. 605, 1872. https://doi.org/10.1039/js8722500605
Tags: Henry Armstrong, Kekule vibration, Paul Schleyer
Posted in Historical | 10 Comments »
May 18th, 2014
Continuing my european visits, here are two photos from Bonn. First, a word about how the representation of benzene evolved, attributed to Kekulé.
Read the rest of this entry »
Tags: /RT, free energy barrier, gas constant
Posted in Historical | 3 Comments »
May 18th, 2014
Not a computer in sight! I refer to a chemistry lab from the 1800s I was recently taken to, where famous french chemists such as Joseph Gay-Lussac, Michel Chevreul and Edmond Fremy were professors. Although not used for chemistry any more, it is an incredible treasure trove of objects. Here are photos of some.
Read the rest of this entry »
Tags: Edmond Fremy, Joseph Gay-Lussac, Michel Chevreul
Posted in Historical | No Comments »
May 17th, 2014
I remember a time when tracking down a particular property of a specified molecule was an all day effort, spent in the central library (or further afield). Then came the likes of STN Online (~1980) and later Beilstein. But only if your institution had a subscription. Let me then cut to the chase: consider this URL: http://search.datacite.org/ui?q=InChIKey%3DLQPOSWKBQVCBKS-PGMHMLKASA-N The site is datacite, which collects metadata about cited data! Most of that data is open in the sense that it can be retrieved without a subscription (but see here that it is not always made easy to do so). So, the above is a search for cited data which contains the InChIkey LQPOSWKBQVCBKS-PGMHMLKASA-N. This produces the result:
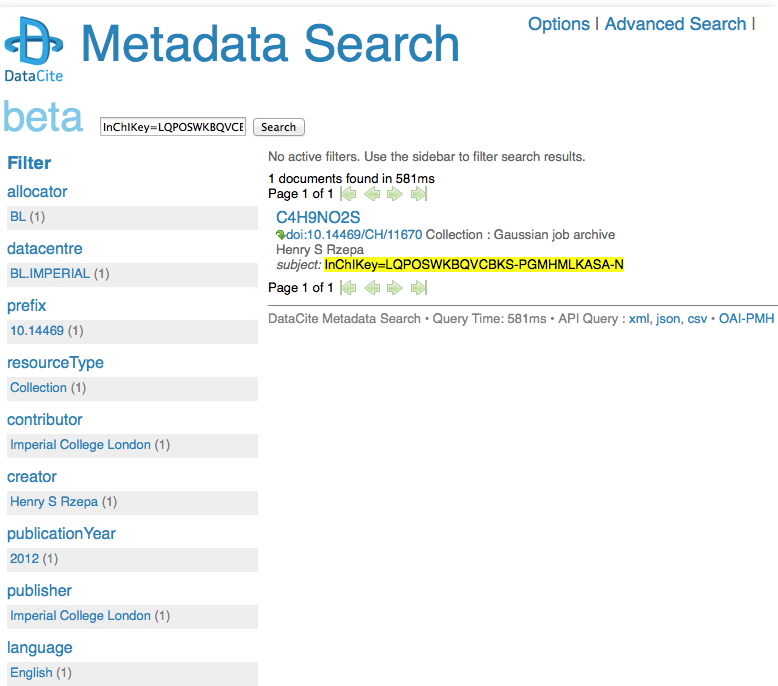
This tells you who published the data (but oddly, its date is merely to the nearest year? It is beta software after all). The advanced equivalent of this search looks like this:
Read the rest of this entry »
Tags: beta software, generic data search, molecular search engines, search engine, search engine optimisation, search looks, software agents
Posted in Chemical IT | No Comments »
May 5th, 2014
My name is displayed pretty prominently on this blog, but it is not always easy to find out who the real person is behind many a blog. In science, I am troubled by such anonymity. Well, a new era is about to hit us. When you come across an Internet resource, or an opinion/review of some scientific topic, I argue here that you should immediately ask: “what is its provenance?”
Read the rest of this entry »
Tags: 0000-0002-8635-8390, added value site, API, Internet resource, ORCiD, programmer, United Kingdom
Posted in Chemical IT | No Comments »


