The last post addressed the concept of “steric clashes” in a pericyclic reaction transition state as an extension of the time honoured practice of building molecular models to analyse reaction outcomes. A modern computer generated model might express this in terms of a NCI (non-covalent-interaction) surface. A few posts ago, I had looked at some “molecules of the year” for 2020, one of which was a “figure-eight” twisted dodecaporphyrin in which an aspect of the reported[cite]10.1038/s41557-019-0398-3[/cite] geometry had struck me as potentially lacking features due to the so-called non-covalent dispersion or van der Waals attractions. So I am revisiting here by adding the NCI surface for this molecule and one other.
Non-covalent-interaction (NCI) surfaces for two large annulenes (revisited).
February 7th, 2021The thermal reactions … took precisely the opposite stereochemical course to that which we had predicted. A non-covalent-interaction view of the model.
February 3rd, 2021Another foray into one of the more famous anecdotal chemistry “models”, the analysis of which led directly to the formulation of the WoodWard-Hoffmann (stereochemical) rules for pericyclic reactions. Previously, I tried to produce a modern computer model of what Woodward might have had to hand when discovering that the stereochemical outcome of a key reaction in his vitamin B12 synthesis was opposite to that predicted using his best model of the reaction.
The Stevens rearrangement: how history gives us new insights.
January 29th, 2021In a recent post, I told the story of how in the early 1960s, Robert Woodward had encountered an unexpected stereochemical outcome to the reaction of a hexatriene, part of his grand synthesis of vitamin B12. He had constructed a model of the reaction he wanted to undertake, perhaps with the help of a physical model, concluding that the most favourable of the two he had built was not matched by the actual outcome of the reaction. He was thus driven to systematise such (Pericyclic) reactions by developing rules for them with Roald Hoffmann. This involved a classification scheme of “allowed” and “forbidden” pericyclic reactions and his original favoured model in fact corresponded to the latter type. When physical model building in the 1960s was gradually replaced by models based on quantum mechanical calculations from the 1970s onwards, the term “allowed” morphed into “a relatively low energy transition state for the reaction can be located” and very often “no transition state exists for a forbidden reaction”. The famous quote “there are no exceptions” (to this rule) was often interpreted that if a “forbidden reaction” did apparently proceed, its mechanism was NOT that of a pericyclic reaction. Inspired by all of this, I recollected a famous “exception” to the rules which is often explained by such non-pericyclic character, the Stevens rearrangement[cite]10.1039/JR9280003193[/cite],[cite]10.1039/JR9300002107[/cite],[cite]10.1039/JR9300002119[/cite] by a 1,2-shift.
The chemical synthesis of C2: another fascinating twist to the story.
January 20th, 2021Last May, I wrote an update to the story sparked by the report of the chemical synthesis of C2.[cite]10.1038/s41467-020-16025-x[/cite] This species has a long history of spectroscopic observation in the gas phase, resulting from its generation at high temperatures.[cite]10.1021/acs.accounts.0c00703[/cite] The chemical synthesis however was done in solution at ambient or low temperatures, a game-changer as they say. Here I give another update to this unfolding story.
The thermal reactions … took precisely the opposite stereochemical course to that which we had predicted
January 20th, 2021The quote of the post title comes from R. B. Woodward explaining the genesis of the discovery of what are now known as the Woodward-Hoffmann rules for pericyclic reactions.[cite]10.1021/ja01080a054[/cite] I first wrote about this in 2012, noting that “for (that) blog, I do not want to investigate the transition states”. Here I take a closer look at this aspect.
Dispersion attraction effects on the computed geometry of a leminscular dodecaporphyrin.
January 1st, 2021In the previous post, I showed the geometries of three large cyclic porphyrins, as part of an article[cite]10.1038/s41557-019-0398-3[/cite] on exploring the aromaticity of large 4n+2 cyclic rings. One of them had been induced into a “figure-eight” or lemniscular conformation, as shown below.
Global aromaticity at the nanoscale.
December 31st, 2020Here is another of the “large” molecules in the c&e news shortlist for molecule-of-the-year, 2020. This one is testing the Hückel 4n+2 rule out to a value never before seen (n = 40, or 162 π-electrons).[cite]10.1038/s41557-019-0398-3[/cite] The take-home message is that this rule seems to behave well in predicting global aromaticity even at this sort of scale!
Tying different knots in a molecular strand.
December 30th, 2020The title derives from an article[cite]10.1038/s41586-020-2614-0[/cite] which was shortlisted for the annual c&en molecule of the year 2020 awards (and which I occasionally cover here). In fact this year’s overall theme is certainly large molecules, the one exception being a smaller molecule with a quadruple bond to boron, a theme I have already covered here.
Is cyanogen chloride (fluoride) a source of C⩸N(+)? More mechanistic insights.
December 4th, 2020I asked the question in my previous post. A computational mechanism revealed that AlCl3 or its dimer Al2Cl6 could catalyse a concerted 1,1-substitution reaction at the carbon of Cl-C≡N, with benzene displacing chloride which is in turn captured by the Al. Unfortunately the calculated barrier for this simple process was too high for a reaction apparently occuring at ~room temperatures. Comments on the post suggested using either a second AlCl3 or a proton to activate the carbon of the C≡N group by coordination on to nitrogen. A second suggestion was to involve di-cationic electrophiles. Here I report the result of implementing the N-coordinated model below.
Is cyanogen chloride (fluoride) a source of C⩸N(+)?
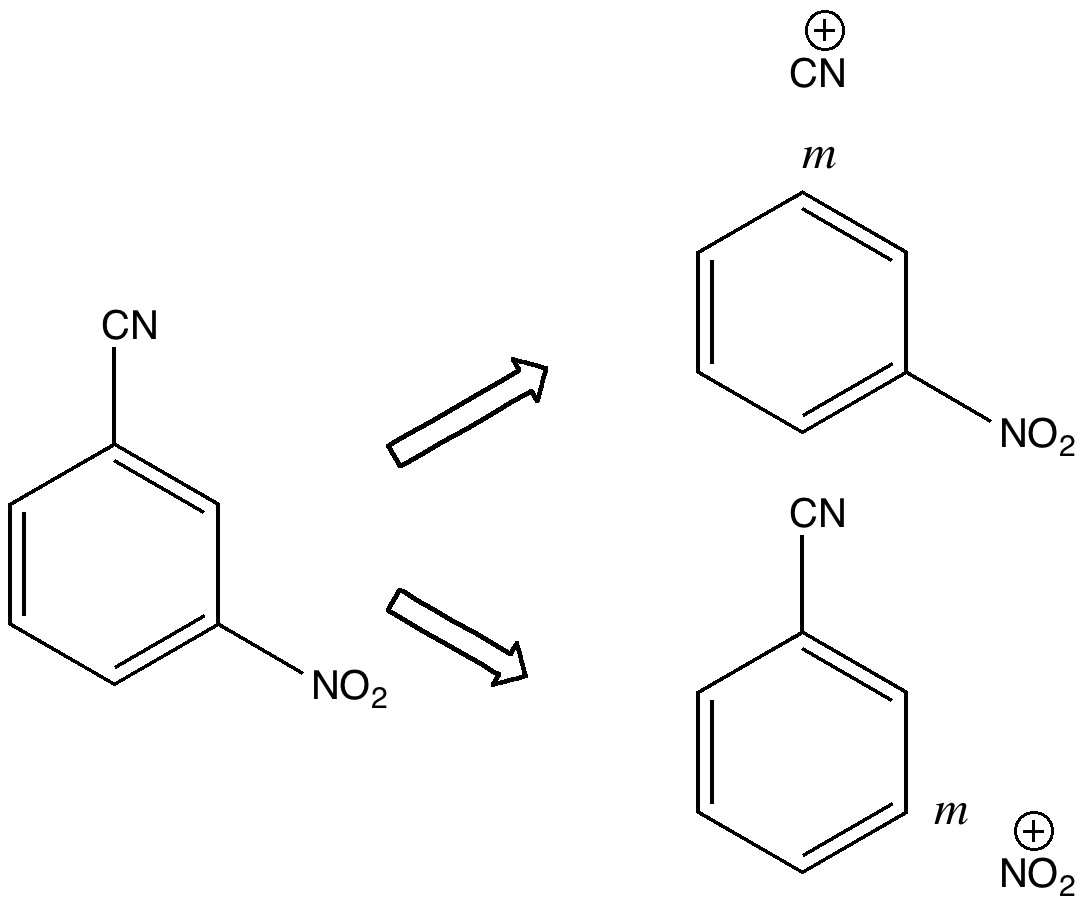
November 28th, 2020In 2010 I recounted the story of an organic chemistry tutorial, in which I asked the students the question “how would you synthesize 3-nitrobenzonitrile“.