On 24th January 1984, the Macintosh computer was released, as all the media are informing us. Apparently, some are still working. I thought I would give my own personal recollections of that period.
The Macintosh computer at 40.
January 25th, 2024A mechanistic exploration of the Wilkinson hydrogenation catalyst. Part 1: Model templates
January 21st, 2024Geoffrey Wilkinson first reported his famous work on the hydrogenation catalyst that now bears his name in 1965[1] and I met him at Imperial College around 1969 and again when I returned there in 1977. He was still working on these catalysts then and I was privileged to collaborate with him on unravelling the NMR spectra of some of these compounds.[2],[3],[4]. During that period, computational modelling of the mechanisms of molecules containing transition elements was still in its infancy and I never extended my collaboration into this area at that time. Now, even if belatedly, I decided to explore this aspect and started to do this about two weeks ago. Here I thought that I would use this opportunity to show how I am going about it.
References
- J.F. Young, J.A. Osborn, F.H. Jardine, and G. Wilkinson, "Hydride intermediates in homogeneous hydrogenation reactions of olefins and acetylenes using rhodium catalysts", Chemical Communications (London), pp. 131, 1965. https://doi.org/10.1039/c19650000131
- K.W. Chiu, H.S. Rzepa, R.N. Sheppard, G. Wilkinson, and W. Wong, "Two-dimensional δ/J-resolved<sup>31</sup>P n.m.r. spectroscopy of [bis(diphenylphosphino)methane](trimethylphosphine)chlororhodium(<scp>I</scp>)", J. Chem. Soc., Chem. Commun., pp. 482-484, 1982. https://doi.org/10.1039/c39820000482
- C. Kwok W., C.G. Howard, H.S. Rzepa, R.N. Sheppard, G. Wilkinson, A.M. Galas, and M.B. Hursthouse, "Trimethyl and diethylphenylphosphine complexes of rhenium(I, III, IV, V) and their reactions. X-ray crystal structures of a bis(η5-cyclopentadienyl)-ethane-bridged dirhenium(I) complex obtained from phenylacetylene, tetrakis-(diethylphenylphosphine) (dinitrogen) hydridorhenium (I), tetrakis(trimethyl-phosphine) (η2-dimethylphosphinomethyl) rhenium(I) and tetrakis(trimethylphosphine) (iodo)methyl rhenium(III) iodide-tetramethylphosphonium iodide", Polyhedron, vol. 1, pp. 441-451, 1982. https://doi.org/10.1016/s0277-5387(00)86558-4
- K.W. Chiu, H.S. Rzepa, R.N. Sheppard, G. Wilkinson, and W. Wong, "Bis(diphenylphosphino)methane trimethylphosphine alkyl and η5-cyclopentadienyl compounds of rhodium(I); 31P{1H} two dimensional δ/J resolved and Overhauser effect nuclear magnetic resonance spectroscopy", Polyhedron, vol. 1, pp. 809-817, 1982. https://doi.org/10.1016/0277-5387(82)80008-9
Scholarly journals vs Scholarly Blogs.
January 12th, 2024First, a very brief history of scholarly publishing, starting in 1665[1] when scientific journals started to be published by learned societies. This model continued until the 1950s, when commercial publishers such as Pergamon Press started with their USP (unique selling point) of rapid time to publication of ~3 months,[2] compared to typical times for many learned society publishers of 2 years or longer. Fast forward another 50 years or so, and the commercial publishers were now dominating the scene, but the business model was still based on institutional subscriptions, whereby the institution rather than authors paid the costs of publication. As the number of journals expanded, even well-off institutions had to make difficult decisions on which subscriptions to keep and which to cancel. By the late 1990s the delivery model was changing from print to online, but the overall issue was that many scientists around the world no longer had access to many journals.
References
- "Epistle dedicatory", Philosophical Transactions of the Royal Society of London, vol. 1, 1665. https://doi.org/10.1098/rstl.1665.0001
- D. Ginsburg, and W.J. Rosenfelder, "Alicyclic studies—X", Tetrahedron, vol. 1, pp. 3-8, 1957. https://doi.org/10.1016/0040-4020(57)85003-0
Macrocyclic peptide antibiotics – now Zosurabalpin – then antibacterial agents based on cyclic D,L-α-peptide architectures.
January 8th, 2024Zosurabalbin[1],[2], is receiving a great deal of attention as a new class of antibiotic which can target infections for which current treatment options are inadequate. It is a cyclic peptide and seeing this triggered memory of an earlier such species reported way back in 1995[3],[4]. This octa-peptide (YIJDIE, DOI: 10.5517/cc58gxs) was presumed to function in a novel manner, having linear water channels wide enough to form a molecular nanoscale pipe for a stream of water molecules to flow along. When inserted into the bacterial cell membrane via its lipophilic sidechains, it drained the bacterium of its cell water within seconds, thus killing it. A 3D model shows the effect very clearly.
References
- C. Zampaloni, P. Mattei, K. Bleicher, L. Winther, C. Thäte, C. Bucher, J. Adam, A. Alanine, K.E. Amrein, V. Baidin, C. Bieniossek, C. Bissantz, F. Boess, C. Cantrill, T. Clairfeuille, F. Dey, P. Di Giorgio, P. du Castel, D. Dylus, P. Dzygiel, A. Felici, F. García-Alcalde, A. Haldimann, M. Leipner, S. Leyn, S. Louvel, P. Misson, A. Osterman, K. Pahil, S. Rigo, A. Schäublin, S. Scharf, P. Schmitz, T. Stoll, A. Trauner, S. Zoffmann, D. Kahne, J.A.T. Young, M.A. Lobritz, and K.A. Bradley, "A novel antibiotic class targeting the lipopolysaccharide transporter", Nature, vol. 625, pp. 566-571, 2024. https://doi.org/10.1038/s41586-023-06873-0
- S. Hawser, N. Kothari, T. Valmont, S. Louvel, and C. Zampaloni, "2131. Activity of the Novel Antibiotic Zosurabalpin (RG6006) against Clinical <i>Acinetobacter</i> Isolates from China", Open Forum Infectious Diseases, vol. 10, 2023. https://doi.org/10.1093/ofid/ofad500.1754
- M.R. Ghadiri, K. Kobayashi, J.R. Granja, R.K. Chadha, and D.E. McRee, "The Structural and Thermodynamic Basis for the Formation of Self‐Assembled Peptide Nanotubes", Angewandte Chemie International Edition in English, vol. 34, pp. 93-95, 1995. https://doi.org/10.1002/anie.199500931
- S. Fernandez-Lopez, H. Kim, E.C. Choi, M. Delgado, J.R. Granja, A. Khasanov, K. Kraehenbuehl, G. Long, D.A. Weinberger, K.M. Wilcoxen, and M.R. Ghadiri, "Antibacterial agents based on the cyclic d,l-α-peptide architecture", Nature, vol. 412, pp. 452-455, 2001. https://doi.org/10.1038/35086601
Molecules of the year 2023 – part 2. A FAIR data comment on a Strontium Metallocene.
December 29th, 2023I will approach this example of a molecule-of-the-year candidate – in fact the eventual winner in the reader poll – from the point of view of data. Its a metallocene arranged in the form of a ring comprising 18 sub-units.[1] Big enough to deserve a 3D model rather than the static images you almost invariably get in journals (and C&EN). So how does one go to the journal and acquire the coordinates for such a model?
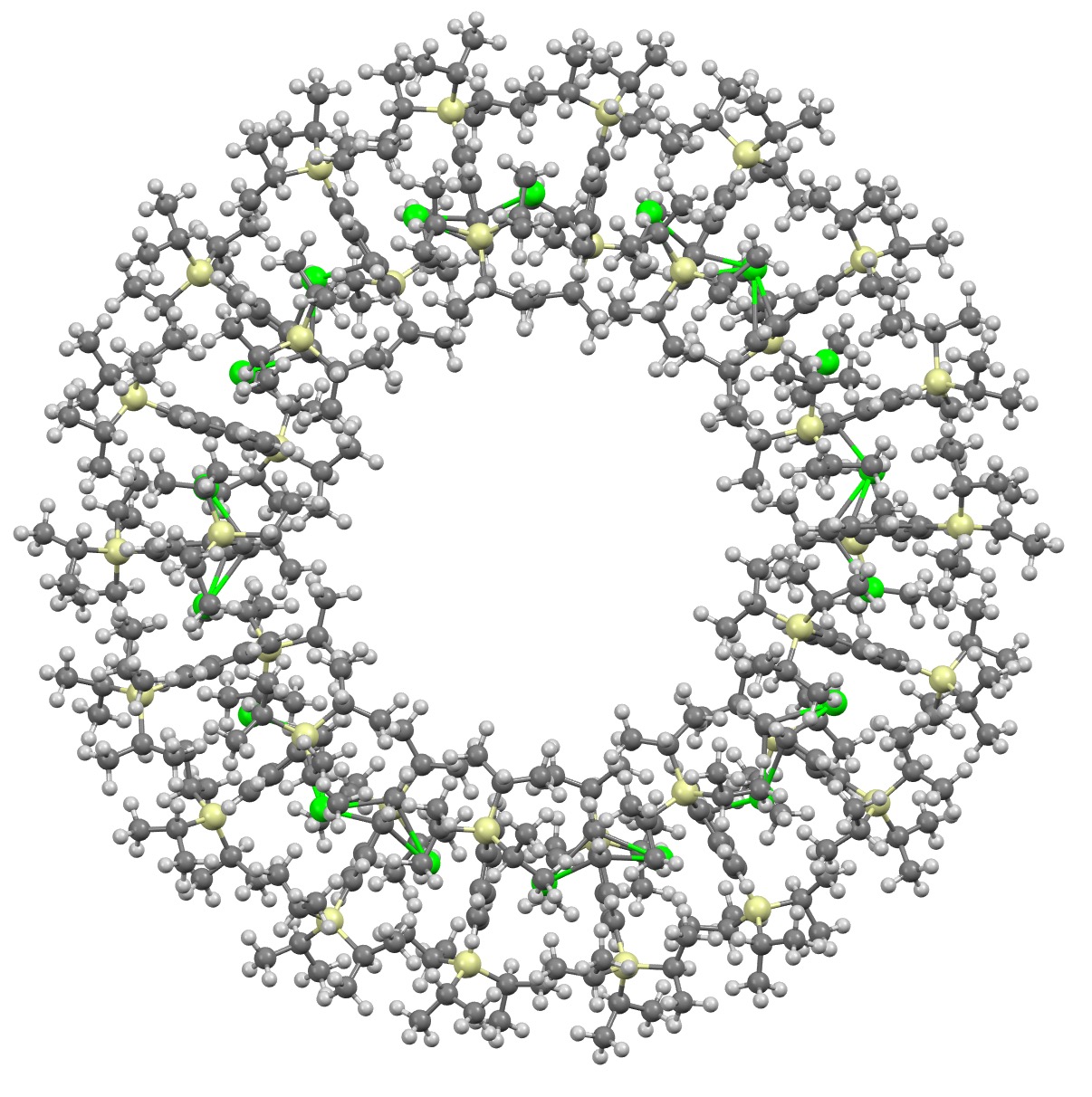
References
- L. Münzfeld, S. Gillhuber, A. Hauser, S. Lebedkin, P. Hädinger, N.D. Knöfel, C. Zovko, M.T. Gamer, F. Weigend, M.M. Kappes, and P.W. Roesky, "Synthesis and properties of cyclic sandwich compounds", Nature, vol. 620, pp. 92-96, 2023. https://doi.org/10.1038/s41586-023-06192-4
Molecules of the year: 2023
December 28th, 2023The Science education unit at the ACS publication C&EN publishes its list of molecules of the year (as selected by the editors and voted upon by the readers) in December. Here are some observations about three of this year’s batch. Read the rest of this entry »
The journey from Journal “ESI” to FAIR data objects: An eighteen year old (continuing) experiment.
December 10th, 2023Around 1996, journals started publishing what became known as “ESI” or electronic supporting information, alongside the articles themselves, as a mechanism for exposing the data associated with the research being reported and exploiting some of the new opportunities offered by the World Wide Web. From the outset, such ESI was expressed as a paginated Acrobat file, with the Web being merely a convenient document delivery mechanism. Such ESI would eventually reach more than 1000 such pages in length in some chemistry articles. The richer opportunities of Web interactivity were far less exploited. I have written about various aspects of this throughout this blog[1],[2],[3], together with one early compendium of our own data examples.[4] Here I update that compendium starting from 2005 to the current 2023 and add further information, being the current state of curation of some of these early examples. Curation became necessary because many of the earlier examples were no longer functional due to changes in the way journals expose these data objects or indeed changes at the data repository end of things over this 18 year period.
References
- H. Rzepa, "Four stages in the evolution of interactive ESI as part of articles in chemistry journals.", 2022. https://doi.org/10.59350/qypm4-qfv97
- H. Rzepa, "Web page decay and Journals: How an interactive "ESI" from 2006 was rescued.", 2022. https://doi.org/10.59350/cqesx-a0e83
- H. Rzepa, "Curating a nine year old journal FAIR data table.", 2017. https://doi.org/10.59350/z9g5j-r2p69
- H. Rzepa, "(Hyper)activating the chemistry journal.", 2009. https://doi.org/10.59350/wczky-8sf79
A trip down memory lane: An online departmental connection map from 1989.
December 7th, 2023In 2023, we very much take for granted that everyone and pretty much everything is online. But it was not always so and when I came across an old plan indicating how the chemistry department at Imperial College was connected in 1989, I was struck by how much has happened in the 34 years since. Nowadays all the infrastructures needed are effectively “built in” to the building when it is constructed and few are even aware of them. But in 1989 that was not at all true.
Two influential textbooks – “Mee” and “Mellor”.
November 11th, 2023I am a member of the Royal Society of Chemistry’s Historical group. Amongst other activities, it publishes two editions of a newsletter each year for its members. A new theme was recently launched asking for contributions on the topic of “two influential books” and shortly to appear in the winter 2023 edition will be the following recollections by myself (reprinted here with permission).
More examples of “double-headed” curly arrows: S and C Nucleophiles attacking acetyl chloride
October 12th, 2023In an earlier post on this topic,[1]‡ I described how the curly-arrows describing the mechanism of a nucleophilic addition at a carbonyl group choreograph in two distinct ways, as seen in red or blue below. The arrows in red can be described as firstly addition to the carbonyl group to form either a transient intermediate (a two-step process) or instead a formal transition state state as a concerted single-step mechanism. The blue arrows do the reverse; firstly elimination and then followed by addition. I will use the shorthand AE for the first type and EA for the second type. Here I explore some more nucleophiles to see which of these two mechanisms they follow. Data for these results can be found at 10.14469/hpc/13171
N- carbon ylid: This is a very facile (low-barrier) reaction with a C-O bond length response that initially increases steeply, followed by a more modest decline and hence corresponds to an AE mechanism.
References
- H. Rzepa, "The "double-headed" curly arrow as used in mechanistic representations.", 2023. https://doi.org/10.59350/f00wf-5tq46