I asked the question in my previous post. A computational mechanism revealed that AlCl3 or its dimer Al2Cl6 could catalyse a concerted 1,1-substitution reaction at the carbon of Cl-C≡N, with benzene displacing chloride which is in turn captured by the Al. Unfortunately the calculated barrier for this simple process was too high for a reaction apparently occuring at ~room temperatures. Comments on the post suggested using either a second AlCl3 or a proton to activate the carbon of the C≡N group by coordination on to nitrogen. A second suggestion was to involve di-cationic electrophiles. Here I report the result of implementing the N-coordinated model below.
Is cyanogen chloride (fluoride) a source of C⩸N(+)?
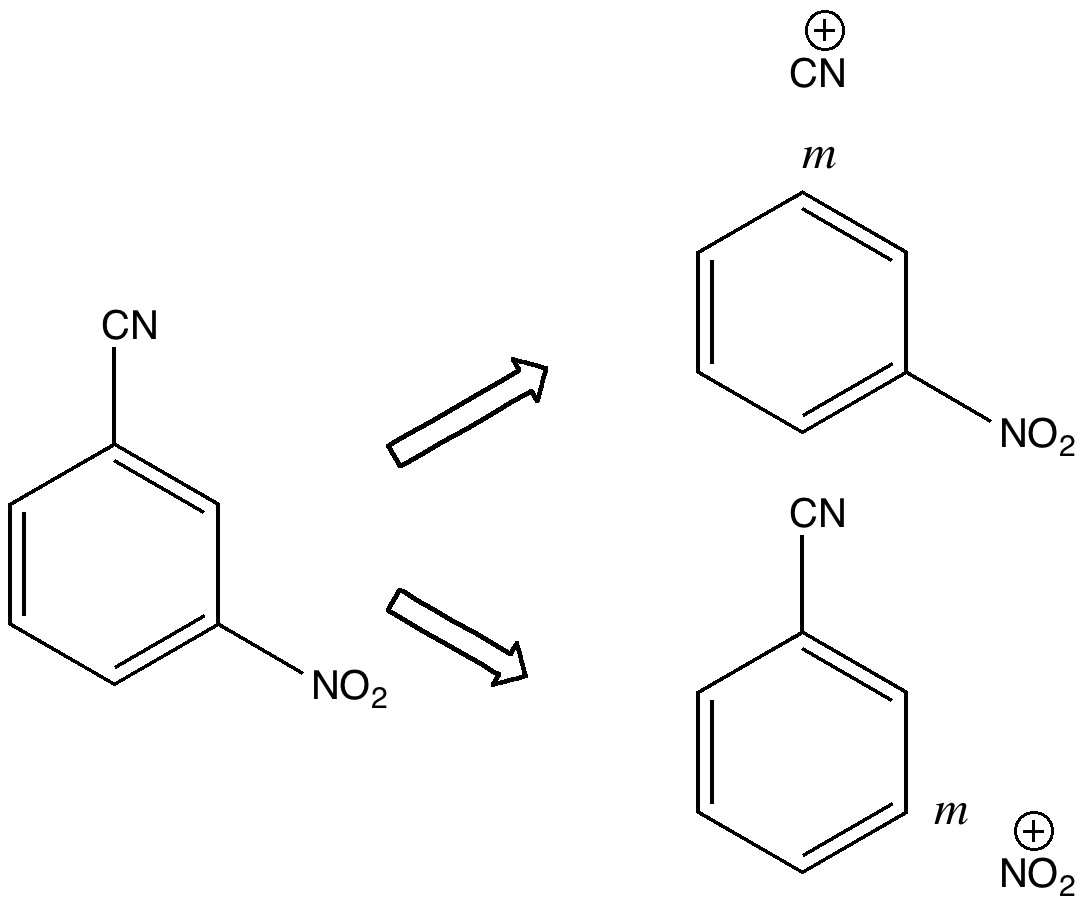
November 28th, 2020In 2010 I recounted the story of an organic chemistry tutorial, in which I asked the students the question “how would you synthesize 3-nitrobenzonitrile“.
An interesting aromatic molecule found in Titan’s atmosphere: Cyclopropenylidene
November 7th, 2020Cyclopropenylidene must be the smallest molecule to be aromatic due to π-electrons, with just three carbon atoms and two hydrogen atoms. It has now been detected in the atmosphere of Titan, one of Saturn’s moons[1] and joins benzene, another aromatic molecule together with the protonated version of cyclopropenylidene, C3H3+ also found there.
References
- C.A. Nixon, A.E. Thelen, M.A. Cordiner, Z. Kisiel, S.B. Charnley, E.M. Molter, J. Serigano, P.G.J. Irwin, N.A. Teanby, and Y. Kuan, "Detection of Cyclopropenylidene on Titan with ALMA", The Astronomical Journal, vol. 160, pp. 205, 2020. https://doi.org/10.3847/1538-3881/abb679
A new example of a quadruple bond from carbon – to Fe.
November 7th, 2020Way back in 2010, I was writing about an experience I had just had during an organic chemistry tutorial, which morphed into speculation as to whether a carbon atom might sustain a quadruple bond to nitrogen. A decade on, and possibly approaching 100 articles by many authors on the topic, quadruple bonds to carbon continue to fascinate. Now an article as appeared[1] repeating this speculation for a carbon to iron quadruple bond,‡ in the very simple species C⩸Fe(CO)3 (see also a Rh-B equivalent[2]). This is particularly exciting because of the very real prospect of synthesising this species and perchance getting a crystal structure (something not possible with most of the other quadruply bonded carbon systems studied to date).
References
- A.J. Kalita, S.S. Rohman, C. Kashyap, S.S. Ullah, and A.K. Guha, "Transition metal carbon quadruple bond: viability through single electron transmutation", Physical Chemistry Chemical Physics, vol. 22, pp. 24178-24180, 2020. https://doi.org/10.1039/d0cp03436c
- L.F. Cheung, T. Chen, G.S. Kocheril, W. Chen, J. Czekner, and L. Wang, "Observation of Four-Fold Boron–Metal Bonds in RhB(BO<sup>–</sup>) and RhB", The Journal of Physical Chemistry Letters, vol. 11, pp. 659-663, 2020. https://doi.org/10.1021/acs.jpclett.9b03484
Internet Archeology: an example of a revitalised molecular resource with a new activity now built in.
November 5th, 2020In Internet terms, 23 years ago is verging on pre-history. Much of what was happening around 1997 on the Web was still highly experimental and so its worth taking a look at some of this to see how it has survived or whether it can be “curated” into a form that would still be useful. I had noted in my earlier comment a site which early on had become non-functional and then speculated whether any volunteers might have suggestions for how to best rescue it.
Trimerous pericyclic reactions: what is the effect of changing the electron count by two?
November 2nd, 2020In an earlier post, I pondered on how the “arrow pushing” for the thermal pericyclic reactions of some annulenes (cyclic conjugated hydrocarbons) could be represented in terms of either two separate electrocyclic reactions or of one cycloaddition reaction. Each reaction is governed by selection rules which can be stated in terms of the anticipated aromaticity of the pericyclic transition state as belonging to a 4n or a 4n+2 class. This in turn determines whether the topology of the transition state belongs to a class of aromatic species known as either Hückel or Möbius. Here I play with the observation that by adding or removing two electrons from the molecule, the two classes 4n and 4n+2 can be swapped. What happens to the aromaticities of the transition states if that is done?
Room-temperature superconductivity in a carbonaceous sulfur hydride!
October 17th, 2020The title of this post indicates the exciting prospect that a method of producing a room temperature superconductor has finally been achived[1]. This is only possible at enormous pressures however; >267 gigaPascals (GPa) or 2,635,023 atmospheres.
References
- E. Snider, N. Dasenbrock-Gammon, R. McBride, M. Debessai, H. Vindana, K. Vencatasamy, K.V. Lawler, A. Salamat, and R.P. Dias, "RETRACTED ARTICLE: Room-temperature superconductivity in a carbonaceous sulfur hydride", Nature, vol. 586, pp. 373-377, 2020. https://doi.org/10.1038/s41586-020-2801-z
Trimerous pericyclic reactions.
October 8th, 2020I occasionally spot an old blog that emerges, if only briefly, as “trending”. In this instance, only the second blog I ever wrote here, way back in 2009 as a follow up to this article.[1] With something of that age, its always worth revisiting to see if any aspect needs updating or expanding, given the uptick in interest. It related to the observation that there can be more than one way of expressing the “curly arrows” for some pericyclic reactions. These alternatives may each represent different types of such reactions, hence leading to a conundrum for students of how to label the mechanism. I had noted in that blog that I intended to revisit the topic and so a mere eleven years later here it is!
References
- H.S. Rzepa, "The Aromaticity of Pericyclic Reaction Transition States", Journal of Chemical Education, vol. 84, pp. 1535, 2007. https://doi.org/10.1021/ed084p1535
Blasts from the past: a snapshot of online content in chemistry, ~1994-1998.
September 28th, 2020With universities around the world having to very rapidly transition to blended learning (a mixture of virtual and face-2-face experiences) with a very large component based on online materials, I thought it might be interesting to try to give one snapshot of when the online experience started to happen in chemistry.
The Willgerodt-Kindler reaction. Completing the Box set.
September 7th, 2020These four posts (the box set) set out to try to define the energetics for a reasonable reaction path for the Willgerodt-Kindler reaction. The rate of this reaction corresponds approximately to a free energy barrier of ~30 kcal/mol. Any pathway found to be >10 kcal/mol at its highest point above this barrier was deemed less probable. The first three efforts at defining such pathways all gave such a result. Here I try a fourth pathway in search of the hitherto elusive appropriately low energy barrier.