The epoxidation of an alkene to give an oxirane is taught in introductory organic chemistry. Formulating an analogous mechanism for such reaction of an alkyne sounds straightforward, but one gradually realises that it requires raiding knowledge from several other areas of (perhaps slightly more advanced) chemistry to achieve a joined up approach to the problem. I had indeed hinted in a previous post that the mechanism for oxidation of acetylene to ketene might be an interesting arrow pushing challenge to set a bright tutorial group, and it was that self-hint that has led me to here. I now explore how my “arrow pushing” intuition stands up to a computational examination.
The peroxidation of alkynes: things are not always what they seem.
November 16th, 2011The dawn of organic reaction mechanism: the prequel.
November 13th, 2011Following on from Armstrong’s almost electronic theory of chemistry in 1887-1890, and Beckmann’s radical idea around the same time that molecules undergoing transformations might do so via a reaction mechanism involving unseen intermediates (in his case, a transient enol of a ketone) I here describe how these concepts underwent further evolution in the early 1920s. My focus is on Edith Hilda Usherwood, who was then a PhD student at Imperial College working under the supervision of Martha Whitely.1
Driving the smallest car ever made: a chemical perspective.
November 10th, 2011Fascination with nano-objects, molecules which resemble every day devices, is increasing. Thus the world’s smallest car has just been built[cite]10.1038/nature10587[/cite]. The mechanics of such a device can often be understood in terms of chemical concepts taught to most students. So I thought I would have a go at this one!
Henry Armstrong: almost an electronic theory of chemistry!
November 7th, 2011Henry Armstrong studied at the Royal College of Chemistry from 1865-7 and spent his subsequent career as an organic chemist at the Central College of the Imperial college of Science and technology until he retired in 1912. He spent the rest of his long life railing against the state of modern chemistry, saving much of his vitriol against (inter alia) the absurdity of ions, electronic theory in chemistry, quantum mechanics and nuclear bombardment in physics. He snarled at Robinson’s and Ingold’s new invention (ca 1926-1930) of electronic arrow pushing with the put down “bent arrows never hit their marks“.‡ He was dismissed as an “old fogy, stuck in a time warp about 1894.”‡ So why on earth would I want to write about him? Read on…
Spotting the unexpected. Anomeric effects involving alkenes?
November 2nd, 2011How one might go about answering the question: do alkenes promote anomeric effects? A search of chemical abstracts does not appear to cite any examples (I may have missed them of course, since it depends very much on the terminology you use, and new effects may not yet have any agreed terminology) and a recent excellent review of hyperconjugation does not mention it. Here I show how one might provide an answer.
Atropisomerism in Taxol. An apparently simple bond rotation?
November 1st, 2011My previous post introduced the interesting guts of taxol. Two different isomers can exist, and these are called atropisomers; one has the carbonyl group pointing up, the other down. The barrier to their interconversion in this case is generated by a rotation about the two single bonds connecting the carbonyl group to the rest of the molecule. Introductory chemistry tells us that the barrier for rotation about such single bonds is low (i.e. fast at room temperature). But is that true here?
Blogbooks, e-books and future proofing chemical diagrams.
October 31st, 2011Most of the chemical structure diagrams in this blog originate from Chemdraw, which seems to have been around since the dawn of personal computers! I have tended to use this program to produce JPG bitmaps for the blog, writing them out in 4x magnification, so that they can be scaled down for display whilst retaining some measure of higher resolution if needed for other purposes. These other purposes might be for e.g. the production of e-books (using Calibre), the interesting Blog(e)book format offered as a service by Feedfabrik, or display on mobile tablets where the touch-zoom metaphor to magnify works particularly well. But bitmap images are not really well future proofed for such new uses. Here I explore one solution to this issue.
Computers 1967-2011: a personal perspective. Part 4. Moore’s Law and Molecules.
October 28th, 2011Moore’s law describes a long-term trend in the evolution of computing hardware, and it is often interpreted in terms of processing speed. Here I chart this rise in terms of the size of computable molecules. By computable I mean specifically how long it takes to predict the geometry of a given molecule using a quantum mechanical procedure.
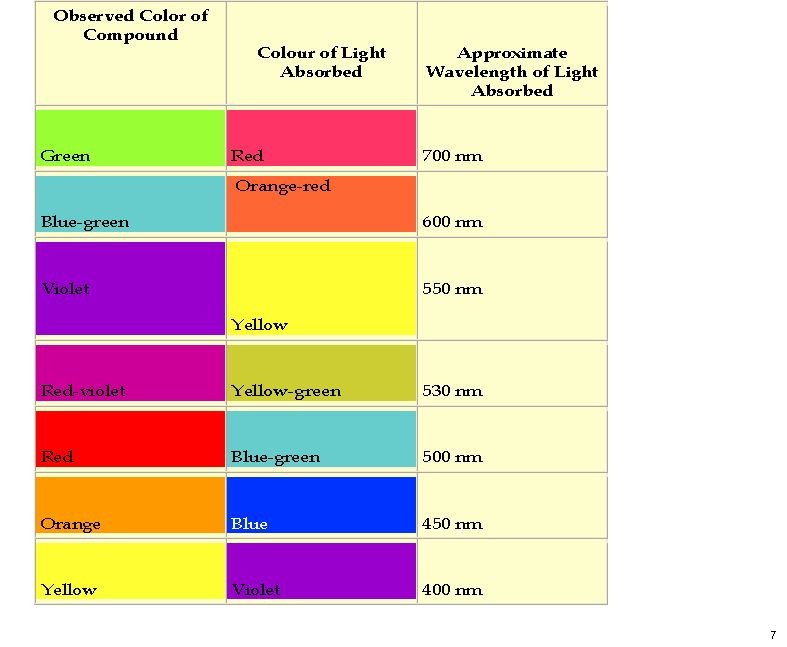
The colour of purple is … not orange but mauve?
October 26th, 2011My previous post on the topic of mauveine left the outcome dangling. Put simply, λmax is measured at about 549nm for mauveine A, but was calculated at about 440nm using a modern method for predicting colour (TD-DFT). According to the colour table below, that would make it orange, not mauve. Can the theoretical prediction be out by 110nm, or might it be the structure of the molecule itself that has been wrongly described?