May 24th, 2010
The assembly of a molecule for a purpose has developed into an art form, one arguably (chemists always argue) that is approaching its 100th birthday (DOI: 10.1002/cber.191104403216) celebrating Willstätter’s report of the synthesis of cyclo-octatetraene. Most would agree it reached its most famous achievement with Woodward’s synthesis of quinine (DOI: 10.1021/ja01221a051) in 1944. To start with, the art was in knowing how and in which order to join up all the bonds of a target. The first synthesis in which (relative) stereocontrol of those bonds was the primary objective was reported in 1951 (10.1021/ja01098a039). The art can be taken one step further. It involves control of the absolute stereochemistry, involving making one enantiomer specifically (rather than the mirror image, which of course has the same relative stereochemistry). Nowadays, a synthesis is considered flawed if the enantiomeric excess (of the desired vs the undesired isomer) of such a synthesis does not achieve at least ~98%. It is routine. But ask the people who design such syntheses if they know exactly the reasons why their reaction has succeeded, you may get a less precise answer (or just a lot of handwaving; chemists also like to wave their hands as well as argue).
Read the rest of this entry »
Tags: energy, free energy, Interesting chemistry, natural product, synthetic chemist
Posted in Interesting chemistry | No Comments »
May 4th, 2010
In this previous blog post I wrote about one way in which we have enhanced the journal article. Associated with that enhancement, and also sprinkled liberally throughout this blog, are links to a Digital Repository (if you want to read all about it, see DOI: 10.1021/ci7004737). It is a fairly specific repository for chemistry, with about 5000 entries. These are mostly the results of quantum mechanical calculations on molecules (together with a much smaller number of spectra, crystal structure and general document depositions). Today, with some help (thanks Matt!), I decided to take a look at how much use the repository was receiving.
Read the rest of this entry »
Tags: chemical repository, Google, Interesting chemistry, Microsoft, opendata, Skolnik, web spiders, Yahoo
Posted in Interesting chemistry | 5 Comments »
May 2nd, 2010
Peter Murray-Rust in his blog asks for examples of the Scientific Semantic Web, a topic we have both been banging on about for ten years or more (DOI: 10.1021/ci000406v). What we are seeking of course is an example of how scientific connections have been made using inference logic from semantically rich statements to be found on the Web (ideally connections that might not have previously been spotted by humans, and lie overlooked and unloved in the scientific literature). Its a tough cookie, and I look forward to the examples that Peter identifies. Meanwhile, I thought I might share here a semantically rich molecule. OK, I identified this as such not by using the Web, but as someone who is in the process of delivering an undergraduate lecture course on the topic of conformational analysis. This course takes the form of presenting a set of rules or principles which relate to the conformations of molecules, and which themselves derive from quantum mechanics, and then illustrating them with selected annotated examples. To do this, a great many semantic connections have to be made, and in the current state of play, only a human can really hope to make most of these. We really look to the semantic web as it currently is to perhaps spot a few connections that might have been overlooked in this process. So, below is a molecule, and I have made a few semantic connections for it (but have not actually fully formalised them in this blog; that is a different topic I might return to at some time). I feel in my bones that more connections could be made, and offer the molecule here as the fuse!
Read the rest of this entry »
Tags: chair, chemical connections, Chemical IT, chemical world, chemist, energy, Fe, General, Interesting chemistry, lowest thermodynamic free energy, organic chemist, organometallic chemist, Peter Murray-Rust, semantic web, unusual
Posted in Chemical IT, General, Interesting chemistry | 2 Comments »
April 18th, 2010
Since I have gotten into the habit of quoting some of my posts in other contexts, I have started to also archive them using WebCite. One can quote the resulting archive as:
Read the rest of this entry »
Tags: archiving, Chemical IT, jmol, Webcite
Posted in Chemical IT | No Comments »
April 16th, 2010
Some molecules, when you first see them, just intrigue. So it was with carbobenzene, the synthesis of a derivative of which was recently achieved by Remi Chauvin and co-workers (DOI: 10.1002/chem.200601193). Two additional carbon atoms have been inserted into each of the six C-C bonds in benzene.
Read the rest of this entry »
Tags: aromatic systems, General, http, Missouri, Remi Chauvin
Posted in General | 4 Comments »
April 6th, 2010
Here I offer another spin-off from writing a lecture course on conformational analysis. This is the famous example of why 1,2-difluoroethane adopts a gauche rather than antiperiplanar conformation.
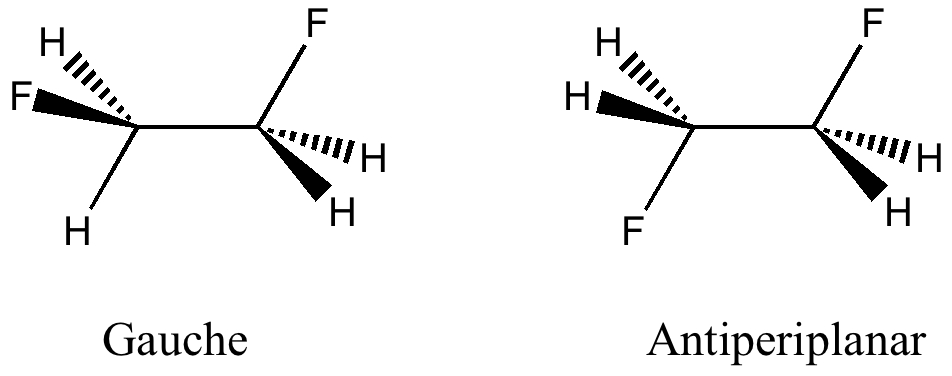
The gauche and antiperiplanar conformations of 1,2-difluoroethane
Read the rest of this entry »
Tags: appropriate algorithms, conformational analysis, energy gap, Here I, interaction energy, Interesting chemistry, spin-off
Posted in Interesting chemistry | 14 Comments »
April 2nd, 2010
One of the (not a few) pleasures of working in a university is the occasional opportunity that arises to give a new lecture course to students. New is not quite the correct word, since the topic I have acquired is Conformational analysis. The original course at Imperial College was delivered by Derek Barton himself about 50 years ago (for articles written by him on the topic, see DOI 10.1126/science.169.3945.539 or the original 10.1039/QR9561000044), and so I have had an opportunity to see how the topic has evolved since then, and perhaps apply some quantitative quantum mechanical interpretations unavailable to Barton himself.
Read the rest of this entry »
Tags: 10.1021, conformational analysis, Derek Barton, energy maxima, Imperial College, Interesting chemistry, lower energy, overall energy, potential energy surface, Tutorial material
Posted in Interesting chemistry | 11 Comments »
March 13th, 2010
One future vision for chemistry over the next 20 years or so is the concept of having machines into which one dials a molecule, and as if by magic, the required specimen is ejected some time later. This is in some ways an extrapolation of the existing peptide and nucleotide synthesizer technologies and sciences. A pretty significant extrapolation, suitable no doubt for a grand future challenge in chemistry (although the concept of tumbling a defined collection of atoms in a computer model and seeing what interesting molecules emerge, dubbed with some sense of humour as mindless chemistry, is already being done; see DOI: 10.1021/jp057107z).
Read the rest of this entry »
Tags: free energy, free energy barrier, Interesting chemistry, metal catalysts, nucleotide synthesizer technologies, similar energy
Posted in Interesting chemistry | No Comments »
February 21st, 2010
Stoyanov, Stoyanova and Reed recently published on the structure of the hydrogen ion in water. Their model was H(H2O)n+, where n=6 (DOI: 10.1021/ja9101826). This suggestion was picked up by Steve Bachrach on his blog, where he added a further three structures to the proposed list, and noted of course that with this type of system there must be a fair chance that the true structure consists of a well-distributed Boltzmann population of a number of almost iso-energetic forms.
Read the rest of this entry »
Tags: animation, gas phase model, General, Interesting chemistry, Steve Bachrach
Posted in General, Interesting chemistry | 1 Comment »
February 20th, 2010
In the previous post, I ruminated about how chemists set themselves targets. Thus, having settled on describing regions between two (and sometimes three) atoms as bonds, they added a property of that bond called its order. The race was then on to find molecules which exhibit the highest order between any particular pair of atoms. The record is thus far five (six has been mooted but its a little less certain) for the molecule below
Read the rest of this entry »
Tags: bonding, ELF, Fluorine, Hiberty and co, Hypervalency
Posted in Hypervalency | 5 Comments »
