August 4th, 2016
Here is a little molecule that can be said to be pretty electron rich. There are lots of lone pairs present, and not a few electron-deficient σ-bonds. I thought it might be fun to look at the stereoelectronic interactions set up in this little system.
Read the rest of this entry »
Tags: chemical bonding, Sigma bond, Stereoelectronic effect, X-ray
Posted in crystal_structure_mining, Interesting chemistry | No Comments »
August 1st, 2016
In March, I posted from the ACS meeting in San Diego on the topic of Research data: Managing spectroscopy-NMR, and noted a talk by MestreLab Research on how a tool called Mpublish in the forthcoming release of their NMR analysis software Mestrenova could help. With that release now out, the opportunity arose to test the system.
Read the rest of this entry »
Tags: Acrobat, analysis software, chemical, Chemistry, City: San Diego, format type chemical/x-mnpub, media type, Mestrenova, non-commercial open software packages, Nuclear magnetic resonance, Nuclear magnetic resonance spectra database, Nuclear magnetic resonance spectroscopy, PDF, public key, Science, Scientific method, spectroscopy, Technology/Internet
Posted in Chemical IT | 3 Comments »
July 8th, 2016
The previous post looked at anomeric effects set up on centres such as B, Si or P, and involving two oxygen groups attached to these atoms. Here I vary the attached groups to include either one or two nitrogen atoms.[cite]10.14469/hpc/936[/cite]
Read the rest of this entry »
Tags: Anomer, Anomeric effect, Carbohydrate chemistry, Carbohydrate conformation, Carbohydrates, Chemistry, Nitrogen
Posted in crystal_structure_mining | No Comments »
July 1st, 2016
The anomeric effect occurs at 4-coordinate (sp3) carbon centres carrying two oxygen substituents and involves an alignment of a lone electron pair on one oxygen with the adjacent C-O σ*-bond of the other oxygen. Here I explore whether other centres can exhibit the phenomenon. I start with 4-coordinate boron, using the crystal structure search definition below (along with R < 0.1, no disorder, no errors).[cite]10.14469/hpc/696[/cite]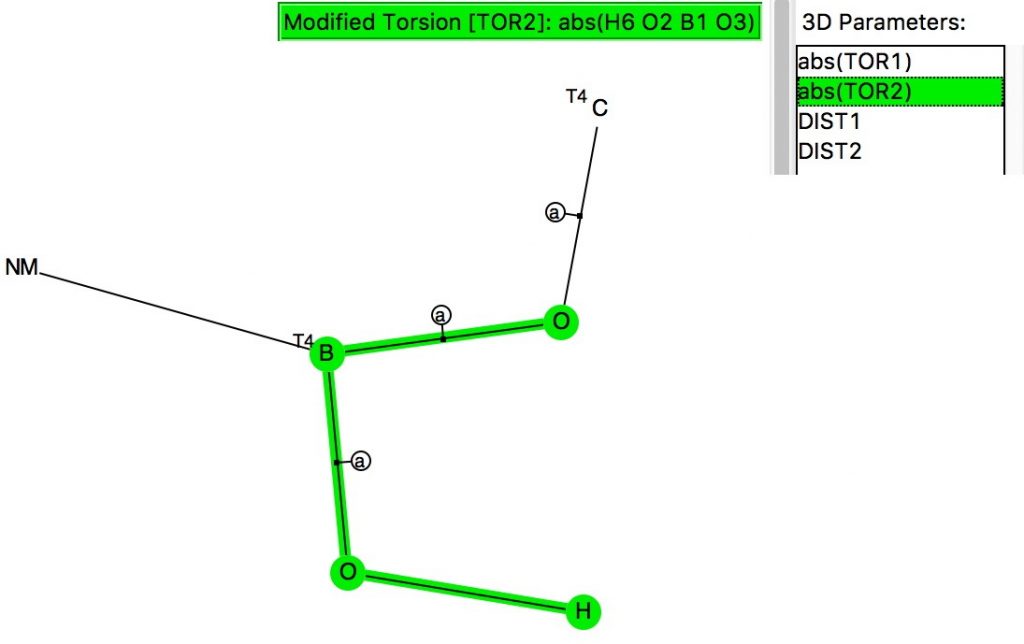
Read the rest of this entry »
Tags: Acetals, Alkane stereochemistry, Anomer, Anomeric effect, Bond length, Boron, Carbohydrate, Carbohydrate chemistry, Carbohydrates, crystal structure search definition, Ester, Physical organic chemistry, Stereochemistry
Posted in crystal_structure_mining | No Comments »
June 22nd, 2016
I previously used data mining of crystal structures to explore the directing influence of substituents on aromatic and heteroaromatic rings. Here I explore, quite literally, a different angle to the hydrogen bonding interactions between a benzene ring and OH or NH groups.
Read the rest of this entry »
Tags: 10.1021, 10.1107, 10.5517, aromaticity, benzene, Centroid, chemical bonding, data mining, Functional groups, Hydrogen bond, Physical organic chemistry, Pyridine, Simple aromatic rings, Supramolecular chemistry
Posted in Chemical IT, crystal_structure_mining | 3 Comments »
June 21st, 2016
This is a follow-up to the post on exploring the directing influence of (electron donating) substituents on benzene[cite]10.1021/acs.jchemed.5b00346[/cite] with the focus on heteroaromatic rings such indoles, pyrroles and group 16 analogues (furans, thiophenes etc).
Read the rest of this entry »
Tags: Asymmetric hydrogenation, benzene, benzo, Electrophile, Furan, Indole, Pyridine, Pyrrole, search query, Simple aromatic rings, Substitution reaction, Thiophene
Posted in crystal_structure_mining | No Comments »
June 18th, 2016
In this post, I pondered upon the C=O infra-red spectroscopic properties of esters, and showed three possible electronic influences:
Read the rest of this entry »
Tags: Ester, Functional groups, Infra-Red
Posted in Chemical IT, crystal_structure_mining | 1 Comment »
June 13th, 2016
Previously, I looked at the historic origins of the so-called π-complex theory of metal-alkene complexes. Here I follow this up with some data mining of the crystal structure database for such structures.
Read the rest of this entry »
Tags: alkene, alkene-metal complex, alkyne, Bond length, Carbon–carbon bond, Chemical bond, chemical bonding, Cluster chemistry, Conquest structure editor, Coordination complex, data mining, double bond, editor, filled metal orbital, metal, metal-alkene complexes, metal-alkyne complexes, metal-carbon bonds, Pi backbonding, search query, Structural formula, Transition metal alkyne complex
Posted in crystal_structure_mining | No Comments »
June 10th, 2016
A while ago, I explored how the 3-coordinate halogen compound ClF3 is conventionally analyzed using VSEPR (valence shell electron pair repulsion theory). Here I (belatedly) look at other such tri-coordinate halogen compounds using known structures gleaned from the crystal structure database (CSD).
Read the rest of this entry »
Tags: chemical phenomena, data mining, equivalent search, Halogen, search query specifies 7A
Posted in crystal_structure_mining | 1 Comment »
June 3rd, 2016
The title might give it away; this is my 500th blog post, the first having come some eight years ago. Very little online activity nowadays is excluded from measurement and so it is no surprise that this blog and another of my "other" scholarly endeavours, viz publishing in traditional journals, attract such "metrics" or statistics. The h-index is a well-known but somewhat controversial measure of the impact of journal articles; here I thought I might instead take a look at three less familiar ones – one relating to blogging, one specific to journal publishing and one to research data.
Read the rest of this entry »
Tags: Country: Svalbard and Jan Mayen, CrossRef, head of information resources, HTML, Imperial College, librarian, online activity, Online Usage, PDF, researcher, search engines, usage statistics portal
Posted in Chemical IT | 4 Comments »
