Six years ago, I posted on the nature of a then recently reported[cite]10.1002/anie.200803859[/cite] Cr-Cr quintuple bond. The topic resurfaced as part of the discussion on a more recent post on NSF3, and a sub-topic on the nature of the higher order bonding in C2. The comment made a connection between that discussion and the Cr-Cr bond alluded to above. I responded briefly to that comment, but because I want to include 3D rotatable surfaces, I expand the discussion here and not in the comment.‡
VSEPR Theory: A closer look at trifluorothionitrile, NSF3.
January 16th, 2016The post on applying VSEPR ("valence shell electron pair repulsion") theory to the geometry of ClF3 has proved perennially popular. So here is a follow-up on another little molecue, F3SN. As the name implies, it is often represented with an S≡N bond. Here I take a look at the conventional analysis.
I’ve started so I’ll finish. Kinetic isotope effect models for a general acid as a catalyst in the protiodecarboxylation of indoles.
January 10th, 2016Earlier I explored models for the heteroaromatic electrophilic protiodecarboxylation of an 3-substituted indole, focusing on the role of water as the proton transfer and delivery agent. Next, came models for both water and the general base catalysed ionization of indolinones. Here I explore general acid catalysis by evaluating the properties of two possible models for decarboxylation of 3-indole carboxylic acid, one involving proton transfer (PT) from neutral water in the presence of covalent un-ionized HCl (1) and one with PT from a protonated water resulting from ionised HCl (2).
Some examples of open access publications citing managed research data (RDM).
January 5th, 2016In May 2015, the EPSRC funding council in the UK required researchers to publish the outcomes of the funded work to include an OA (open access) version of the narrative and to cite the managed research data used to support the research with a DOI (digital object identifier). I was discussing these aspects with a senior manager (research outcomes) at the EPSRC and he asked me to provide some examples from my area of chemistry; here are some.
I’ve started so I’ll finish. The mechanism of diazo coupling to indoles – forty (three) years on!
December 24th, 2015
The BBC TV quiz series Mastermind was first broadcast in the UK in 1972, the same time I was starting to investigate the mechanism of diazocoupling to substituted indoles as part of my Ph.D. researches. The BBC program became known for the catch phrase I've started so I'll finish; here I will try to follow this precept with the project I started then. In 1972, one measured the rates of chemical reactions to gain insights into the transition state kinetic model. To obtain more data, we used isotopes such as 2H or 3H, together with substituents such as R-t-butyl to modify the potential energy surfaces of the reactions by inducing steric effects.[cite]10.1039/P29750001209[/cite],[cite]10.5281/zenodo.18777[/cite] We found that the kinetics for this reaction were actually complex‡ (in part because of pH dependence) involving a Wheland intermediate (the formation of which is shown with red curly arrows above) followed by the collapse of this intermediate to the diazo-coupled product (blue arrows). Coupling to 2-methyl indole (R=X=H, R'=Me), 2-t-butyl indole (R=H, R'=t-butyl) and 4-methyl-2-t-butyl indole (R=Me, R'=t-butyl) revealed that the kinetic isotope effects induced by replacing H by D or T were "not apparent" (i.e. close to 1), the inference being that the rate constant k1 for those systems was slower than k2; the formation of the Wheland intermediate was rate determining (the rds) for the reaction. But with 2-methyl-4,6-di-t-butyl indole (R=t-butyl, R'=Me) this changed and a deuterium isotope effect of ~7 was observed. The rate determining proton removal from the Wheland intermediate k2 was now slower than k1. With 2,4,6-tri-t-butyl indole, we ended by noting that the reaction become almost too slow to observe and furthermore was accompanied by loss of a t-butyl cation as well as a proton. At this point we attempted to infer some transition state models consistent with these observations. Note that we had relatively little data with which to derive our 3D models (one needs to define a geometry using 3N-6 variables, along with its relative energy and force constants). The text and diagram of our attempt is shown below.
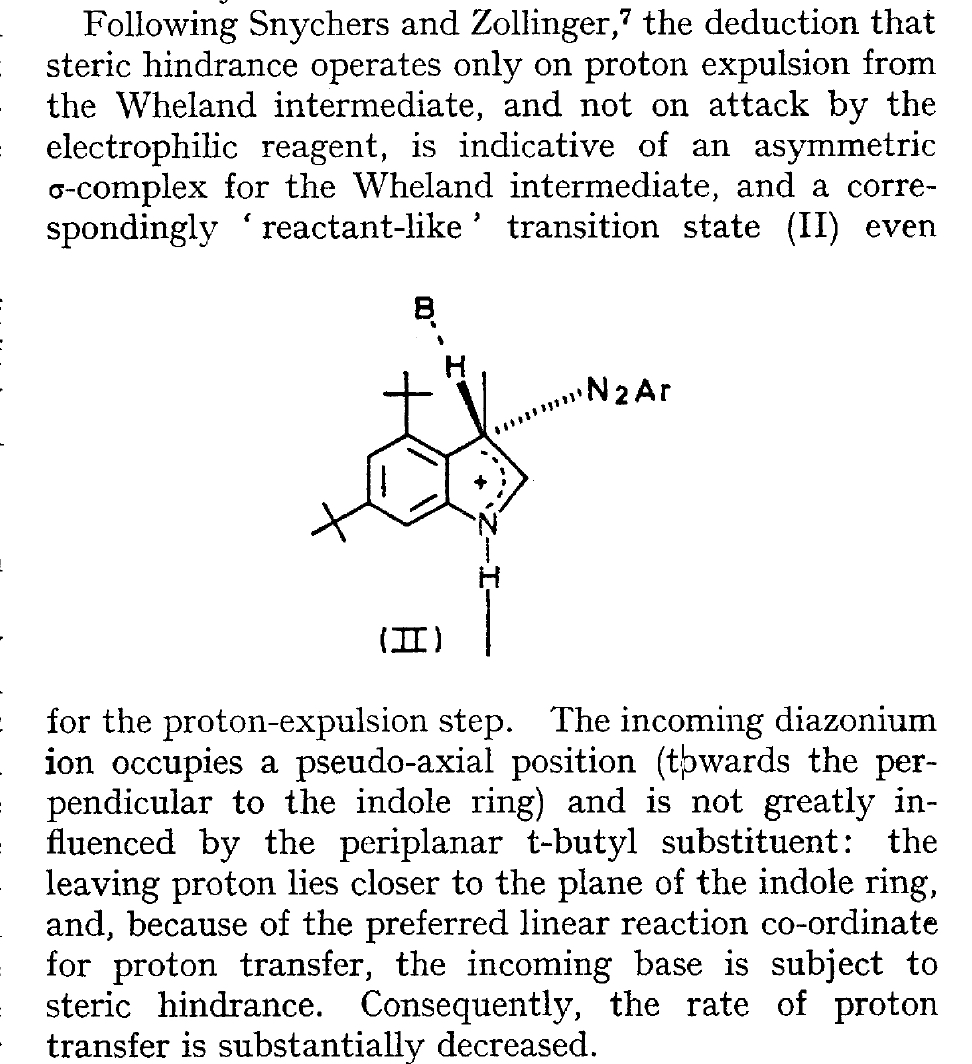 The main points of this argument were;
The main points of this argument were;
Could anyone comment on any recent calculated results on the planarity, or lack thereof, of azobenzene?
December 20th, 2015The atom and the molecule: A one-day symposium on 23 March, 2016 celebrating Gilbert N. Lewis.
December 11th, 2015You might have noticed the occasional reference here to the upcoming centenary of the publication of Gilbert N. Lewis’ famous article entitled “The atom and the molecule“.[cite]10.1021/ja02261a002[/cite] A symposium exploring his scientific impact and legacy will be held in London on March 23, 2016, exactly 70 years to the day since his death. A list of the speakers and their titles is shown below; there is no attendance fee, but you must register as per the instructions below.