No doubt answers to the question posed in the previous post are already being obtained by experiment. Just in case that does not emerge in the next day or so, I offer a prediction here.
How many water molecules does it take to ionise HF and HBr?
February 27th, 2015How many water molecules does it take to ionise HCl?
February 14th, 2015According to Guggemos, Slavicek and Kresin, about 5-6![cite]10.1103/PhysRevLett.114.043401[/cite]. This is one of those simple ideas, which is probably quite tough to do experimentally. It involved blasting water vapour through a pinhole, adding HCl and measuring the dipole-moment induced deflection by an electric field. They found “evidence for a noticeable rise in the dipole moment occurring at n≈5–6“.
How-open-is-it?
February 12th, 2015The title of this post refers to the site http://howopenisit.org/ which is in effect a license scraper for journal articles. In the past 2-3 years in the UK, we have been able to make use of grants to our university to pay publishers to convert our publications into Open Access (also called GOLD). I thought I might check out a few of my recent publications to see what http://howopenisit.org/ makes of them.
Chiroptical spectroscopy of the natural product Steganone.
February 10th, 2015Steganone is an unusual natural product, known for about 40 years now. The assignment of its absolute configurations makes for an interesting, on occasion rather confusing, and perhaps not entirely atypical story. I will start with the modern accepted stereochemical structure of this molecule, which comes in the form of two separately isolable atropisomers.
The first reported synthesis of this system in 1977 was racemic, and no stereochemistry is shown in the article (structure 2).[cite]10.1039/P19770001674[/cite] Three years later an “Asymmetric total synthesis of (-)steganone and revision of its absolute configuration” shows how the then accepted configuration (structure 1 in this article) needs to be revised to the enantiomer shown as structure 12 in the article[cite]10.1016/S0040-4039(00)78586-8[/cite] and matching the above representation. The system has continued to attract interest ever since[cite]10.1039/P19820000521[/cite],[cite]10.1039/A900743A[/cite],[cite]10.1039/C39950001943[/cite],[cite]10.1002/ejoc.201402761[/cite], not least because of the presence of axial chirality in the form of atropisomerism. Thus early on it was shown that the alternative atropisomer, the (aS,R,R) configuration initially emerges out of several syntheses, and has to be converted to the (aR,R,R) configuration by heating[cite]10.1039/P19820000521[/cite]. One could easily be fooled by such isomerism!
Mechanism of the solvatochromic reaction of a spiropyran.
February 4th, 2015The journal of chemical education has many little gems providing inspiration for laboratory experiments. Jonathan Piard reports one based on the reaction below[cite]10.1021/ed4005003[/cite]; here I investigate the mechanism of this transformation.
Fine-tuning a (hydrogen) bond into symmetry.
January 23rd, 2015Sometimes you come across a bond in chemistry that just shouts at you. This happened to me in 1989[cite]10.1039/C39890001722[/cite] with the molecule shown below. Here is its story and, 26 years later, how I responded.
A convincing example of the need for data repositories. FAIR Data.
January 15th, 2015Derek Lowe in his In the Pipeline blog is famed for spotting unusual claims in the literature and subjecting them to analysis. This one is entitled Odd Structures, Subjected to Powerful Computations. He looks at this image below, and finds the structures represented there might be a mistake, based on his considerable experience of these kinds of molecules. I expect he had a gut feeling within seconds of seeing the diagram.
The demographics of a blog readership – updated
January 8th, 2015About two years ago, I posted on the distribution of readership of this blog. The passage of time has increased this from 144 to 176 countries. There are apparently between 189-196 such, so not quite yet complete coverage! 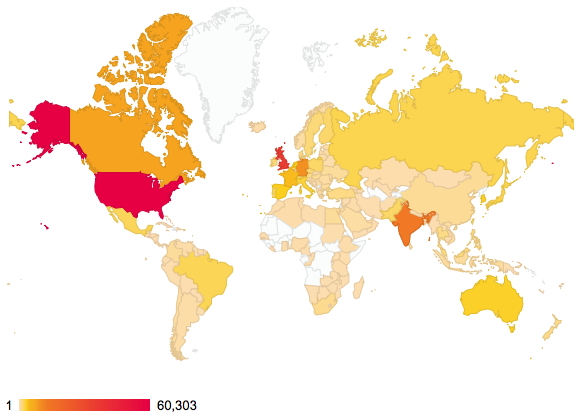
Of course, it is the nature of the beast that whilst we can track countries, very little else is known about such readerships. Is the readership young or old, student or professor, chemist or not (although I fancy the latter is less likely). Another way of keeping tabs on some of the activity are aggregators such as Chemical Blogspace, which has been rather quiet recently. Perhaps we have become too obsessed by metrics, and with the Internet-of-things apparently the “next-big-thing”, the metrics are only likely to increase. This will only encourage “game playing“, and I urge you to see a prime example of this in the UK REF (research excellence framework), the measure which attempts to rank UK universities in terms of their “excellence”.
Chemistry in the early 1960s: a reminiscence.
December 22nd, 2014I started chemistry with a boxed set in 1962. In those days they contained serious amounts of chemicals, but I very soon ran out of most of them. Two discoveries turned what might have been a typical discarded christmas present into a lifelong career and hobby.
Data discoverability
December 17th, 2014I have written earlier about the Amsterdam Manifesto. That arose out of a conference on the theme of “beyond the PDF“, with one simple question at its heart: what can be done to liberate data from containers it was not designed to be in? The latest meeting on this topic will happen in January 2015 as FORCE2015.