February 10th, 2015
Steganone is an unusual natural product, known for about 40 years now. The assignment of its absolute configurations makes for an interesting, on occasion rather confusing, and perhaps not entirely atypical story. I will start with the modern accepted stereochemical structure of this molecule, which comes in the form of two separately isolable atropisomers.

The first reported synthesis of this system in 1977 was racemic, and no stereochemistry is shown in the article (structure 2).[1] Three years later an “Asymmetric total synthesis of (-)steganone and revision of its absolute configuration” shows how the then accepted configuration (structure 1 in this article) needs to be revised to the enantiomer shown as structure 12 in the article[2] and matching the above representation. The system has continued to attract interest ever since[3],[4],[5],[6], not least because of the presence of axial chirality in the form of atropisomerism. Thus early on it was shown that the alternative atropisomer, the (aS,R,R) configuration initially emerges out of several syntheses, and has to be converted to the (aR,R,R) configuration by heating[3]. One could easily be fooled by such isomerism!
Read the rest of this entry »
References
- D. Becker, L.R. Hughes, and R.A. Raphael, "Total synthesis of the antileukaemic lignan (±)-steganacin", J. Chem. Soc., Perkin Trans. 1, pp. 1674-1681, 1977. https://doi.org/10.1039/p19770001674
- J. Robin, O. Gringore, and E. Brown, "Asymmetric total synthesis of the antileukaemic lignan precursor (-)steganone and revision of its absolute configuration", Tetrahedron Letters, vol. 21, pp. 2709-2712, 1980. https://doi.org/10.1016/s0040-4039(00)78586-8
- E.R. Larson, and R.A. Raphael, "Synthesis of (–)-steganone", J. Chem. Soc., Perkin Trans. 1, pp. 521-525, 1982. https://doi.org/10.1039/p19820000521
- A. Bradley, W.B. Motherwell, and F. Ujjainwalla, "A concise approach towards the synthesis of steganone analogues", Chemical Communications, pp. 917-918, 1999. https://doi.org/10.1039/a900743a
- M. Uemura, A. Daimon, and Y. Hayashi, "An asymmetric synthesis of an axially chiral biaryl via an (arene)chromium complex: formal synthesis of (–)-steganone", J. Chem. Soc., Chem. Commun., vol. 0, pp. 1943-1944, 1995. https://doi.org/10.1039/c39950001943
- B. Yalcouye, S. Choppin, A. Panossian, F.R. Leroux, and F. Colobert, "A Concise Atroposelective Formal Synthesis of (–)‐Steganone", European Journal of Organic Chemistry, vol. 2014, pp. 6285-6294, 2014. https://doi.org/10.1002/ejoc.201402761
Tags: free energy, lowest energy conformation, natural product, simulation, spectroscopy, unusual natural product, X-ray
Posted in Interesting chemistry | No Comments »
February 4th, 2015
The journal of chemical education has many little gems providing inspiration for laboratory experiments. Jonathan Piard reports one based on the reaction below[1]; here I investigate the mechanism of this transformation.
Read the rest of this entry »
References
- J. Piard, "Influence of the Solvent on the Thermal Back Reaction of One Spiropyran", Journal of Chemical Education, vol. 91, pp. 2105-2111, 2014. https://doi.org/10.1021/ed4005003
Tags: calculated free energy, chemical education, Jonathan Piard
Posted in reaction mechanism | No Comments »
January 23rd, 2015
Sometimes you come across a bond in chemistry that just shouts at you. This happened to me in 1989[1] with the molecule shown below. Here is its story and, 26 years later, how I responded.
Read the rest of this entry »
References
- P. Camilleri, C.A. Marby, B. Odell, H.S. Rzepa, R.N. Sheppard, J.J.P. Stewart, and D.J. Williams, "X-Ray crystallographic and NMR evidence for a uniquely strong OH ? N hydrogen bond in the solid state and solution", Journal of the Chemical Society, Chemical Communications, pp. 1722, 1989. https://doi.org/10.1039/c39890001722
Tags: energy, high chemical shifts, New Hampshire, unusual chemical shift
Posted in Historical, Interesting chemistry | No Comments »
January 8th, 2015
About two years ago, I posted on the distribution of readership of this blog. The passage of time has increased this from 144 to 176 countries. There are apparently between 189-196 such, so not quite yet complete coverage! 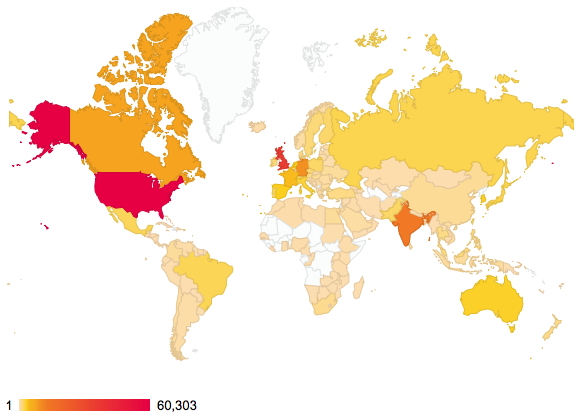
Of course, it is the nature of the beast that whilst we can track countries, very little else is known about such readerships. Is the readership young or old, student or professor, chemist or not (although I fancy the latter is less likely). Another way of keeping tabs on some of the activity are aggregators such as Chemical Blogspace, which has been rather quiet recently. Perhaps we have become too obsessed by metrics, and with the Internet-of-things apparently the “next-big-thing”, the metrics are only likely to increase. This will only encourage “game playing“, and I urge you to see a prime example of this in the UK REF (research excellence framework), the measure which attempts to rank UK universities in terms of their “excellence”.
Read the rest of this entry »
Tags: chemist, Internet-of-things, professor, United Kingdom
Posted in General | 2 Comments »
December 22nd, 2014
I started chemistry with a boxed set in 1962. In those days they contained serious amounts of chemicals, but I very soon ran out of most of them. Two discoveries turned what might have been a typical discarded christmas present into a lifelong career and hobby.
Read the rest of this entry »
Tags: A. N. Beck and Sons, Albert N. Beck, chemical stains, chemicals, chemist, christmas, Craven Cottage, GBP, London, pence, Shilling
Posted in Historical | 57 Comments »
December 17th, 2014
I have written earlier about the Amsterdam Manifesto. That arose out of a conference on the theme of “beyond the PDF“, with one simple question at its heart: what can be done to liberate data from containers it was not designed to be in? The latest meeting on this topic will happen in January 2015 as FORCE2015.
Read the rest of this entry »
Posted in Chemical IT | 4 Comments »
December 14th, 2014
These posts contain the computed potential energy surfaces for a fair few “text-book” reactions. Here I chart the course of the cyclopropanation of alkenes using the Simmons-Smith reagent,[1] as prepared from di-iodomethane using zinc metal insertion into a C-I bond.

Read the rest of this entry »
References
- H.E. Simmons, and R.D. Smith, "A NEW SYNTHESIS OF CYCLOPROPANES FROM OLEFINS", Journal of the American Chemical Society, vol. 80, pp. 5323-5324, 1958. https://doi.org/10.1021/ja01552a080
Tags: computed potential energy surfaces, di-iodomethane using zinc metal insertion, Simmons
Posted in reaction mechanism | 1 Comment »
December 7th, 2014
Continuing my hunt, here is a candidate for a strong(est?) halogen bond, this time between Se and I.[1]. 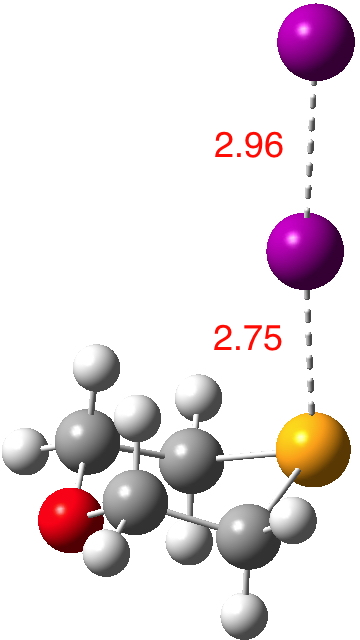 The features of interest include:
The features of interest include:
Read the rest of this entry »
References
- H. Maddox, and J.D. McCullough, "The Crystal and Molecular Structure of the Iodine Complex of 1-Oxa-4-selenacyclohexane, C<sub>4</sub>H<sub>8</sub>OSe.I<sub>2</sub>", Inorganic Chemistry, vol. 5, pp. 522-526, 1966. https://doi.org/10.1021/ic50038a006
Tags: chair, crystal structure search
Posted in crystal_structure_mining, Interesting chemistry | 7 Comments »
December 1st, 2014
Nitrogen tri-iodide, or more accurately the complex between it and ammonia ranks amongst the oldest known molecules (1812). I became familiar with it around the age of 12-13, in an era long gone when boys (and very possibly girls too) were allowed to make such substances in their parent’s back gardens‡ and in fact in the school science laboratory,† an experiment which earned me a personal request to visit the head teacher.
Read the rest of this entry »
Tags: full periodic boundary model, head teacher, teacher, the unit
Posted in Interesting chemistry | No Comments »

