Text books (is this a misnomer, much like “papers” are in journals?) in a higher-educational chemistry environment, I feel, are at a cross-roads. What happens next?
Posts Tagged ‘energy’
Stereoselectivities of Proline-Catalyzed Asymmetric Intermolecular Aldol Reactions.
Sunday, April 22nd, 2012Astronomers who discover an asteroid get to name it, mathematicians have theorems named after them. Synthetic chemists get to name molecules (Hector’s base and Meldrum’s acid spring to mind) and reactions between them. What do computational chemists get to name? Transition states! One of the most famous of recent years is the Houk-List.
An exothermic E2 elimination: an unusual intrinsic reaction coordinate.
Monday, February 6th, 2012The previous post explored why E2 elimination reactions occur with an antiperiplanar geometry for the transition state. Here I have tweaked the initial reactant to make the overall reaction exothermic rather than endothermic as it was before. The change is startling.
A modern take on pericyclic cycloaddition. Dimerisation of cis-butene
Monday, November 28th, 2011The π2 + π2 cyclodimerisation of cis-butene is the simplest cycloaddition reaction with stereochemical implications. I here give it the same treatment as I did previously for electrocyclic pericyclic reactions.
The dawn of organic reaction mechanism: the prequel.
Sunday, November 13th, 2011Following on from Armstrong’s almost electronic theory of chemistry in 1887-1890, and Beckmann’s radical idea around the same time that molecules undergoing transformations might do so via a reaction mechanism involving unseen intermediates (in his case, a transient enol of a ketone) I here describe how these concepts underwent further evolution in the early 1920s. My focus is on Edith Hilda Usherwood, who was then a PhD student at Imperial College working under the supervision of Martha Whitely.1
Driving the smallest car ever made: a chemical perspective.
Thursday, November 10th, 2011Fascination with nano-objects, molecules which resemble every day devices, is increasing. Thus the world’s smallest car has just been built[1]. The mechanics of such a device can often be understood in terms of chemical concepts taught to most students. So I thought I would have a go at this one!
References
- T. Kudernac, N. Ruangsupapichat, M. Parschau, B. Maciá, N. Katsonis, S.R. Harutyunyan, K. Ernst, and B.L. Feringa, "Electrically driven directional motion of a four-wheeled molecule on a metal surface", Nature, vol. 479, pp. 208-211, 2011. https://doi.org/10.1038/nature10587
Computers 1967-2011: a personal perspective. Part 4. Moore’s Law and Molecules.
Friday, October 28th, 2011Moore’s law describes a long-term trend in the evolution of computing hardware, and it is often interpreted in terms of processing speed. Here I chart this rise in terms of the size of computable molecules. By computable I mean specifically how long it takes to predict the geometry of a given molecule using a quantum mechanical procedure.
The colour of purple is … not orange but mauve?
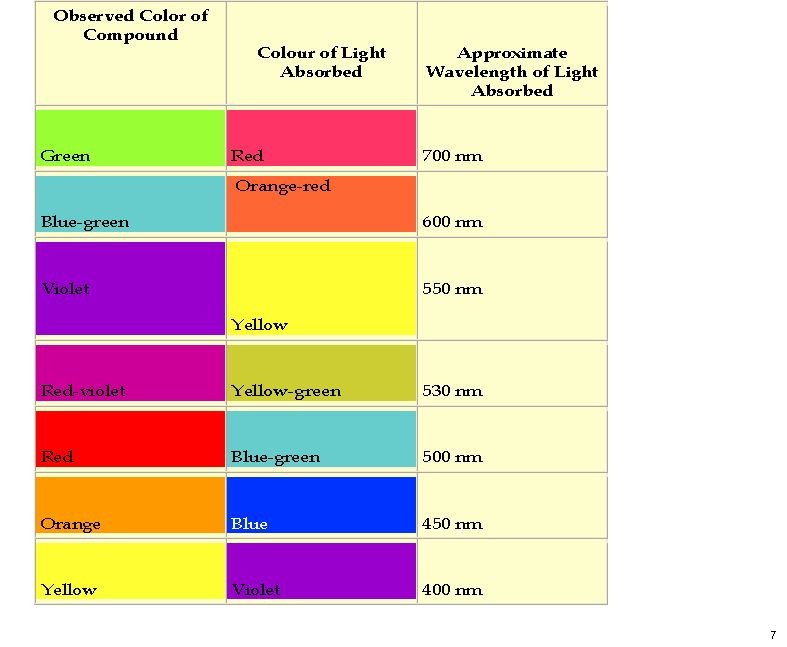
Wednesday, October 26th, 2011My previous post on the topic of mauveine left the outcome dangling. Put simply, λmax is measured at about 549nm for mauveine A, but was calculated at about 440nm using a modern method for predicting colour (TD-DFT). According to the colour table below, that would make it orange, not mauve. Can the theoretical prediction be out by 110nm, or might it be the structure of the molecule itself that has been wrongly described?
Are close H…H contacts bonds? The dénouement!
Monday, October 10th, 2011I wrote earlier about the strangely close contact between two hydrogen atoms in cis-butene. The topology of the electron density showed characteristics of a bond, but is it a consensual union? The two hydrogens approach closer than their van der Waals radii would suggest is normal, so something is happening, but that something need not be what chemists might choose to call a “bond“. An NCI (non-covalent analysis) hinted that any stability due to the electron topologic characteristics of a bond (the BCP) might be more than offset by the repulsive nature of the adjacent ring critical point (RCP). Here I offer an alternative explanation for why the two hydrogens approach so closely.
Are close H…H contacts bonds?
Friday, October 7th, 2011The properties of electrons are studied by both chemists and physicists. At the boundaries of these two disciplines, sometimes interesting differences in interpretation emerge. One of the most controversial is that due to Bader (for a recent review, see DOI: 10.1021/jp102748b) a physicist who brought the mathematical rigor of electronic topology to bear upon molecules. The title of his review is revealing: “Definition of Molecular Structure: By Choice or by Appeal to Observation?”. He argues that electron density is observable, and that what chemists call a bond should be defined by that observable (with the implication that chemists instead often resort to arbitrary choice). Here I explore one molecule which could be said to be the focus of the differences between physics and chemistry; cis-but-2-ene.