December 22nd, 2014
I started chemistry with a boxed set in 1962. In those days they contained serious amounts of chemicals, but I very soon ran out of most of them. Two discoveries turned what might have been a typical discarded christmas present into a lifelong career and hobby.
Read the rest of this entry »
Tags: A. N. Beck and Sons, Albert N. Beck, chemical stains, chemicals, chemist, christmas, Craven Cottage, GBP, London, pence, Shilling
Posted in Historical | 55 Comments »
December 17th, 2014
I have written earlier about the Amsterdam Manifesto. That arose out of a conference on the theme of “beyond the PDF“, with one simple question at its heart: what can be done to liberate data from containers it was not designed to be in? The latest meeting on this topic will happen in January 2015 as FORCE2015.
Read the rest of this entry »
Posted in Chemical IT | 4 Comments »
December 14th, 2014
These posts contain the computed potential energy surfaces for a fair few “text-book” reactions. Here I chart the course of the cyclopropanation of alkenes using the Simmons-Smith reagent,[cite]10.1021/ja01552a080[/cite] as prepared from di-iodomethane using zinc metal insertion into a C-I bond.

Read the rest of this entry »
Tags: computed potential energy surfaces, di-iodomethane using zinc metal insertion, Simmons
Posted in reaction mechanism | 1 Comment »
December 7th, 2014
Continuing my hunt, here is a candidate for a strong(est?) halogen bond, this time between Se and I.[cite]10.1021/ic50038a006[/cite]. 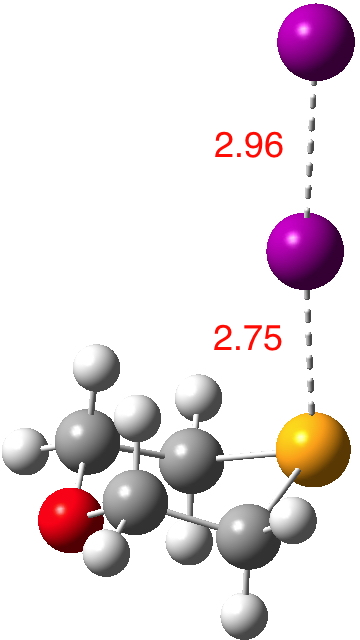 The features of interest include:
The features of interest include:
Read the rest of this entry »
Tags: chair, crystal structure search
Posted in crystal_structure_mining, Interesting chemistry | 7 Comments »
December 1st, 2014
Nitrogen tri-iodide, or more accurately the complex between it and ammonia ranks amongst the oldest known molecules (1812). I became familiar with it around the age of 12-13, in an era long gone when boys (and very possibly girls too) were allowed to make such substances in their parent’s back gardens‡ and in fact in the school science laboratory,† an experiment which earned me a personal request to visit the head teacher.
Read the rest of this entry »
Tags: full periodic boundary model, head teacher, teacher, the unit
Posted in Interesting chemistry | No Comments »
November 30th, 2014
Pursuing the topic of halogen bonds, the system DABCO (a tertiary dibase) and iodine form an intriguing complex. Here I explore some unusual features of the structure HEKZOO[cite]10.5517/CCYJN03[/cite] as published in 2012[cite]10.1021/cg300669t[/cite] and ask whether the bonding between the donor (N) and the acceptor (I-I) really is best described as a “non-covalent-interaction” (NCI) or not.
Read the rest of this entry »
Tags: bond energy, co-operative, donor-acceptor interaction energy
Posted in Interesting chemistry | 5 Comments »
November 29th, 2014
Halogen bonds are less familiar cousins to hydrogen bonds. They are defined as non-covalent interactions (NCI) between a halogen atom (X, acting as a Lewis acid, in accepting electrons) and a Lewis base D donating electrons; D….X-A vs D…H-A. They are superficially surprising, since both D and X look like electron rich species. In fact the electron distribution around X-X (A=X) is highly anisotropic, with the electron rich distribution (the "donor") being in a torus encircling the bond, and an electron deficient region (the "acceptor") lying along the axis of the bond.
Read the rest of this entry »
Tags: crystal structure search, D. Note, frequent commentator, Paul Schleyer
Posted in crystal_structure_mining, Interesting chemistry, reaction mechanism | No Comments »
November 12th, 2014
In London, one has the pleasures of attending occasional one day meetings at the Burlington House, home of the Royal Society of Chemistry. On November 5th this year, there was an excellent meeting on the topic of Challenges in Catalysis, and you can see the speakers and (some of) their slides here. One talk on the topic of Direct amide formation – the issues, the art, the industrial application by Dave Jackson caught my interest. He asked whether an amide could be formed directly from a carboxylic acid and an amine without the intervention of an explicit catalyst. The answer involved noting that the carboxylic acid was itself a catalyst in the process, and a full mechanistic exploration of this aspect can be found in an article published in collaboration with Andy Whiting's group at Durham.[cite]10.1002/ejoc.201100714[/cite] My after-thoughts in the pub centered around the recollection that I had written some blog posts about the reaction between hydroxylamine and propanone. Might there be any similarity between the two mechanisms?
Read the rest of this entry »
Tags: Andy Whiting, Dave Jackson, dielectric, Durham, energy profile, free energy barrier, London, non-polar solution, PDF, Royal Society of Chemistry
Posted in reaction mechanism | 6 Comments »
November 6th, 2014
Solvolytic mechanisms are amongst the oldest studied, but reproducing their characteristics using computational methods has been a challenging business. This post was inspired by reading Steve Bachrach’s post, itself alluding to this aspect in the title “Computationally handling ion pairs”. It references this recent article on the topic[cite]10.1021/jo501012s[/cite] in which the point is made that reproducing the features of both contact and solvent-separated ion pairs needs a model comprising discrete solvent molecules (in this case four dichloromethane units) along with a continuum model.
Read the rest of this entry »
Tags: 10.1021, Steve Bachrach
Posted in reaction mechanism | No Comments »
November 1st, 2014
Egon Willighagen recently gave a presentation at the RSC entitled “The Web – what is the issue” where he laments how little uptake of web technologies as a “channel for communication of scientific knowledge and data” there is in chemistry after twenty years or more. It caused me to ponder what we were doing with the web twenty years ago. Our HTTP server started in August 1993, and to my knowledge very little content there has been deleted (it’s mostly now just hidden). So here are some ancient pages which whilst certainly not examples of how it should be done nowadays, give an interesting historical perspective. In truth, there is not much stuff that is older out there!
Read the rest of this entry »
Tags: 3D printing language, ACS, BBC, Bryan Levitt, chemical audience, chemical information technologies, Darek Bogdal, Fortran, Guillaume Cottenceau, HTML, http, Java, large supermarket chain, personal Web presence, Python, researcher, spectroscopy, Tesco, Virtual reality, WATOC, web technologies
Posted in Chemical IT, Historical | No Comments »
 The features of interest include:
The features of interest include:
Halogen bonds: Part 1.
November 29th, 2014Halogen bonds are less familiar cousins to hydrogen bonds. They are defined as non-covalent interactions (NCI) between a halogen atom (X, acting as a Lewis acid, in accepting electrons) and a Lewis base D donating electrons; D….X-A vs D…H-A. They are superficially surprising, since both D and X look like electron rich species. In fact the electron distribution around X-X (A=X) is highly anisotropic, with the electron rich distribution (the "donor") being in a torus encircling the bond, and an electron deficient region (the "acceptor") lying along the axis of the bond.
Read the rest of this entry »
Tags: crystal structure search, D. Note, frequent commentator, Paul Schleyer
Posted in crystal_structure_mining, Interesting chemistry, reaction mechanism | No Comments »