The previous post looked at anomeric effects set up on centres such as B, Si or P, and involving two oxygen groups attached to these atoms. Here I vary the attached groups to include either one or two nitrogen atoms.[cite]10.14469/hpc/936[/cite]
Anomeric effects at carbon involving lone pairs originating from one or two nitrogens.
July 8th, 2016Anomeric effects at boron, silicon and phosphorus.
July 1st, 2016
The anomeric effect occurs at 4-coordinate (sp3) carbon centres carrying two oxygen substituents and involves an alignment of a lone electron pair on one oxygen with the adjacent C-O σ*-bond of the other oxygen. Here I explore whether other centres can exhibit the phenomenon. I start with 4-coordinate boron, using the crystal structure search definition below (along with R < 0.1, no disorder, no errors).[cite]10.14469/hpc/696[/cite]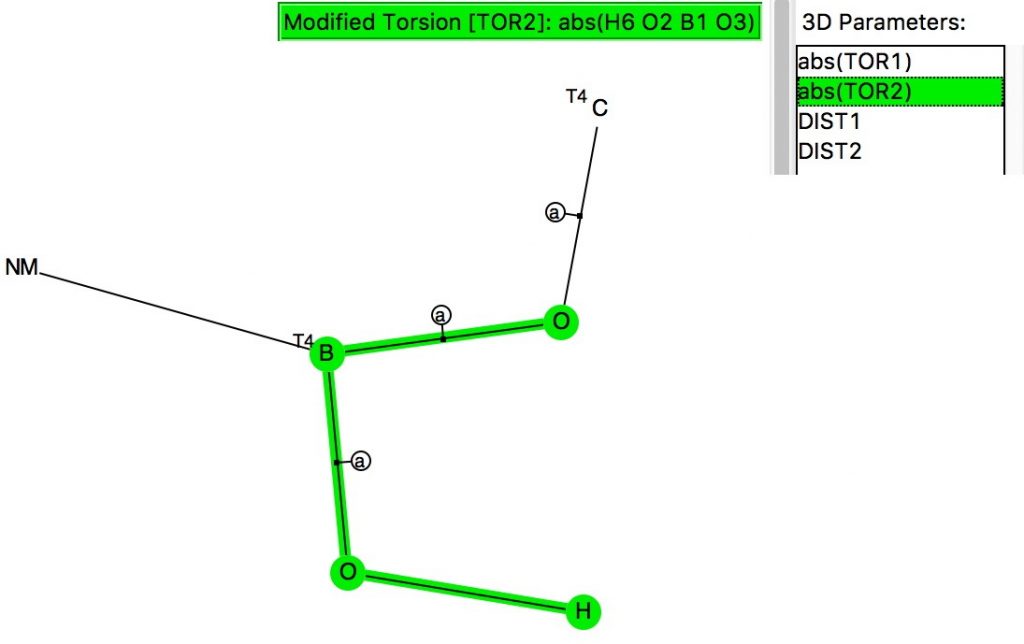
How does an OH or NH group approach an aromatic ring to hydrogen bond with its π-face?
June 22nd, 2016I previously used data mining of crystal structures to explore the directing influence of substituents on aromatic and heteroaromatic rings. Here I explore, quite literally, a different angle to the hydrogen bonding interactions between a benzene ring and OH or NH groups.
Exploring the electrophilic directing influence of heteroaromatic rings using crystal structure data mining.
June 21st, 2016This is a follow-up to the post on exploring the directing influence of (electron donating) substituents on benzene[cite]10.1021/acs.jchemed.5b00346[/cite] with the focus on heteroaromatic rings such indoles, pyrroles and group 16 analogues (furans, thiophenes etc).
Why is the carbonyl IR stretch in an ester higher than in a ketone: crystal structure data mining.
June 18th, 2016In this post, I pondered upon the C=O infra-red spectroscopic properties of esters, and showed three possible electronic influences:
A wider look at chlorine trifluoride: crystal structures and data mining.
June 10th, 2016A while ago, I explored how the 3-coordinate halogen compound ClF3 is conventionally analyzed using VSEPR (valence shell electron pair repulsion theory). Here I (belatedly) look at other such tri-coordinate halogen compounds using known structures gleaned from the crystal structure database (CSD).
The geometries of 5-coordinate compounds of group 14 elements.
May 30th, 2016This is a follow-up to one aspect of the previous two posts dealing with nucleophilic substitution reactions at silicon. Here I look at the geometries of 5-coordinate compounds containing as a central atom 4A = Si, Ge, Sn, Pb and of the specific formula C34AO2 with a trigonal bipyramidal geometry. This search arose because of a casual comment I made in the earlier post regarding possible cooperative effects between the two axial ligands (the ones with an angle of ~180 degrees subtended at silicon). Perhaps the geometries might expand upon this comment?
An alternative mechanism for nucleophilic substitution at silicon using a tetra-alkyl ammonium fluoride.
May 27th, 2016In the previous post, I explored the mechanism for nucleophilic substitution at a silicon centre proceeding via retention of configuration involving a Berry-like pseudorotation. Here I probe an alternative route involving inversion of configuration at the Si centre. Both stereochemical modes are known to occur, depending on the leaving group, solvent and other factors.[cite]10.1016/S0040-4020(01)89077-3[/cite],[cite]10.1021/ja01006a024[/cite],[cite]10.1021/ja00784a081[/cite]