The BBC TV quiz series Mastermind was first broadcast in the UK in 1972, the same time I was starting to investigate the mechanism of diazocoupling to substituted indoles as part of my Ph.D. researches. The BBC program became known for the catch phrase I've started so I'll finish; here I will try to follow this precept with the project I started then. In 1972, one measured the rates of chemical reactions to gain insights into the transition state kinetic model. To obtain more data, we used isotopes such as 2H or 3H, together with substituents such as R-t-butyl to modify the potential energy surfaces of the reactions by inducing steric effects.[cite]10.1039/P29750001209[/cite],[cite]10.5281/zenodo.18777[/cite] We found that the kinetics for this reaction were actually complex‡ (in part because of pH dependence) involving a Wheland intermediate (the formation of which is shown with red curly arrows above) followed by the collapse of this intermediate to the diazo-coupled product (blue arrows). Coupling to 2-methyl indole (R=X=H, R'=Me), 2-t-butyl indole (R=H, R'=t-butyl) and 4-methyl-2-t-butyl indole (R=Me, R'=t-butyl) revealed that the kinetic isotope effects induced by replacing H by D or T were "not apparent" (i.e. close to 1), the inference being that the rate constant k1 for those systems was slower than k2; the formation of the Wheland intermediate was rate determining (the rds) for the reaction. But with 2-methyl-4,6-di-t-butyl indole (R=t-butyl, R'=Me) this changed and a deuterium isotope effect of ~7 was observed. The rate determining proton removal from the Wheland intermediate k2 was now slower than k1. With 2,4,6-tri-t-butyl indole, we ended by noting that the reaction become almost too slow to observe and furthermore was accompanied by loss of a t-butyl cation as well as a proton. At this point we attempted to infer some transition state models consistent with these observations. Note that we had relatively little data with which to derive our 3D models (one needs to define a geometry using 3N-6 variables, along with its relative energy and force constants). The text and diagram of our attempt is shown below.
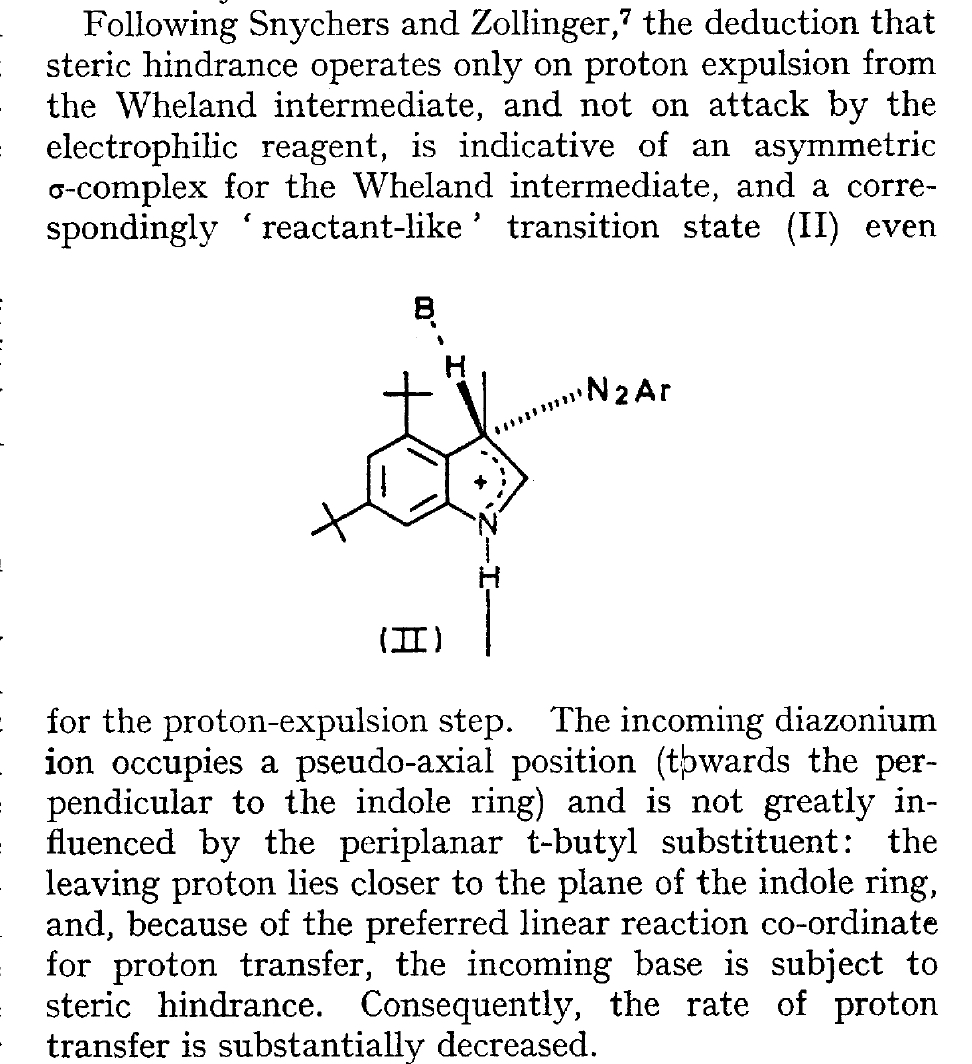 The main points of this argument were;
The main points of this argument were;
I’ve started so I’ll finish. The mechanism of diazo coupling to indoles – forty (three) years on!
December 24th, 2015Could anyone comment on any recent calculated results on the planarity, or lack thereof, of azobenzene?
December 20th, 2015The atom and the molecule: A one-day symposium on 23 March, 2016 celebrating Gilbert N. Lewis.
December 11th, 2015You might have noticed the occasional reference here to the upcoming centenary of the publication of Gilbert N. Lewis’ famous article entitled “The atom and the molecule“.[cite]10.1021/ja02261a002[/cite] A symposium exploring his scientific impact and legacy will be held in London on March 23, 2016, exactly 70 years to the day since his death. A list of the speakers and their titles is shown below; there is no attendance fee, but you must register as per the instructions below.
More stereo electronics: the Eschenmoser double fragmentation and guerrilla tutorials.
December 10th, 2015The layout of floor 2 of the chemistry department here contains a number of small rooms which function as tutorial areas. Each has a (non-interactive) whiteboard used by students and tutors for, inter-alia, thought-showering. It was in one such room that I found myself with three colleagues this monday afternoon. We soon all sensed something not quite right about the room; it slowly dawned that the whiteboard was entirely devoid of thoughts (it is normally left adorned with chemical hieroglyphics). Before we departed, one of our number crept up to the board and showered the following (the red bit only followed by a ?; thanks Willie!). The chemistry equivalent you might say of Guerrilla gardening. The product shown in blue below is for your benefit here. It is an example of a double fragmentation reaction; by an odd coincidence following on nicely from the previous post.
A tutorial problem in stereoelectronic control. A Grob alternative to the Tiffeneau-Demjanov rearrangement?
November 28th, 2015In answering tutorial problems, students often need skills in deciding how much time to spend on explaining what does not happen, as well as what does. Here I explore alternatives to the mechanism outlined in the previous post to see what computation has to say about what does (or might) not happen.
A tutorial problem in stereoelectronic control. The Tiffeneau-Demjanov rearrangement as part of a prostaglandin synthesis.
November 23rd, 2015This reaction emerged a few years ago (thanks Alan!) as a tutorial problem in organic chemistry, in which students had to devise a mechanism for the reaction and use this to predict the stereochemical outcome at the two chiral centres indicated with *. It originates in a brief report from R. B. Woodward’s group in 1973 describing a prostaglandin synthesis,[cite]10.1021/ja00801a066[/cite] the stereochemical outcome being crucial. Here I take a look at this mechanism using computation.
The roles of water in the hydrolysis of an acetal.
November 18th, 2015In the previous post, I pondered how a substituent (X below) might act to slow down the hydrolysis of an acetal. Here I extend that by probing the role of water molecules in the mechanism of acetal hydrolysis.
How to stop (some) acetals hydrolysing.
November 12th, 2015Derek Lowe has a recent post entitled "Another Funny-Looking Structure Comes Through". He cites a recent medchem article[cite]10.1021/acsmedchemlett.5b00398[/cite] in which the following acetal sub-structure appears in a promising drug candidate (blue component below). His point is that orally taken drugs have to survive acid (green below) encountered in the stomach, and acetals are famously sensitive to hydrolysis (red below). But if X=NH2, compound "G-5555" is apparently stable to acids.[cite]10.1021/acsmedchemlett.5b00398[/cite] So I pose the question here; why?
Interactions responsible for the lowest energy structure of the trimer of fluoroethanol.
October 23rd, 2015Steve Bachrach on his own blog has commented on a recent article[cite]10.1002/anie.201505934[/cite] discussing the structure of the trimer of fluoroethanol. Rather than the expected triangular form with three OH—O hydrogen bonds, the lowest energy form only had two such bonds, but it matched the microwave data much better. Here I explore this a bit more.
Pierre and Marie Curie.
October 23rd, 2015I have previously shown the grave of William Perkin, a great british organic chemist. On a recent visit to Paris, I went to see the crypt in the Panthéon, the great french secular necropolis. What a contrast to Perkin!