This is rather cranking the handle, but taking my previous post and altering the search definition of the crystal structure database from 4- to 5-coordinate metals, one gets the following.
Ribulose-1,5-bisphosphate + carbon dioxide → carbon fixation!
April 20th, 2014Ribulose-1,5-bisphosphate reacts with carbon dioxide to produce 3-keto-2-carboxyarabinitol 1,5-bisphosphate as the first step in the biochemical process of carbon fixation. It needs an enzyme to do this (Ribulose-1,5-bisphosphate carboxylase/oxygenase, or RuBisCO) and lots of ATP (adenosine triphosphate, produced by photosynthesis). Here I ask what the nature of the uncatalysed transition state is, and hence the task that might be facing the catalyst in reducing the activation barrier to that of a facile thermal reaction. I present my process in the order it was done‡.
More (blog) connections spotted. Something new about diphenyl magnesium?
April 17th, 2014I have just noticed unexpected links between two old posts, one about benzene, one about diphenyl magnesium and a link to August Kekulé.†
Enantioselective epoxidation of alkenes using the Shi Fructose-based catalyst. An undergraduate experiment.
April 15th, 2014The journal of chemical education can be a fertile source of ideas for undergraduate student experiments. Take this procedure for asymmetric epoxidation of an alkene.[1] When I first spotted it, I thought not only would it be interesting to do in the lab, but could be extended by incorporating some modern computational aspects as well.
References
- A. Burke, P. Dillon, K. Martin, and T.W. Hanks, "Catalytic Asymmetric Epoxidation Using a Fructose-Derived Catalyst", Journal of Chemical Education, vol. 77, pp. 271, 2000. https://doi.org/10.1021/ed077p271
Artemisinin: are stereo-electronics at the core of its (re)activity?
April 13th, 2014Around 100 tons of the potent antimalarial artemisinin is produced annually; a remarkable quantity given its very unusual and fragile looking molecular structure (below). When I looked at this, I was immediately struck by a thought: surely this is a classic molecule for analyzing stereoelectronic effects (anomeric and gauche). Here this aspect is explored.
A connected world (journals and blogs): The benzene dication.
April 10th, 2014Science is rarely about a totally new observation or rationalisation, it is much more about making connections between known facts, and perhaps using these connections to extrapolate to new areas (building on the shoulders of giants, etc). So here I chart one example of such connectivity over a period of six years.
What is the best way of folding a straight chain alkane?
April 6th, 2014In the previous post, I showed how modelling of unbranched alkenes depended on dispersion forces. When these are included, a bent (single-hairpin) form of C58H118 becomes lower in free energy than the fully extended linear form. Here I try to optimise these dispersion forces by adding further folds to see what happens.
Modelling the geometry of unbranched alkanes.
March 29th, 2014By about C17H36, the geometry of “cold-isolated” unbranched saturated alkenes is supposed not to contain any fully anti-periplanar conformations. [1] Indeed, a (co-crystal) of C16H34 shows it to have two-gauche bends.[2]. Surprisingly, the longest linear alkane I was able to find a crystal structure for, C28H58 appears to be fully extended[3],[4] (an early report of a low quality structure for C36H74[5] also appears to show it as linear).‡ Here I explore how standard DFT theories cope with these structures.
References
- N.O.B. Lüttschwager, T.N. Wassermann, R.A. Mata, and M.A. Suhm, "The Last Globally Stable Extended Alkane", Angewandte Chemie International Edition, vol. 52, pp. 463-466, 2012. https://doi.org/10.1002/anie.201202894
- N. Cocherel, C. Poriel, J. Rault‐Berthelot, F. Barrière, N. Audebrand, A. Slawin, and L. Vignau, "New 3π‐2Spiro Ladder‐Type Phenylene Materials: Synthesis, Physicochemical Properties and Applications in OLEDs", Chemistry – A European Journal, vol. 14, pp. 11328-11342, 2008. https://doi.org/10.1002/chem.200801428
- S.C. Nyburg, and A.R. Gerson, "Crystallography of the even <i>n</i>-alkanes: structure of C<sub>20</sub>H<sub>42</sub>", Acta Crystallographica Section B Structural Science, vol. 48, pp. 103-106, 1992. https://doi.org/10.1107/s0108768191011059
- R. Boistelle, B. Simon, and G. Pèpe, "Polytypic structures of n-C28H58 (octacosane) and n-C36H74 (hexatriacontane)", Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry, vol. 32, pp. 1240-1243, 1976. https://doi.org/10.1107/s0567740876005025
- H.M.M. Shearer, and V. Vand, "The crystal structure of the monoclinic form of n-hexatriacontant", Acta Crystallographica, vol. 9, pp. 379-384, 1956. https://doi.org/10.1107/s0365110x5600111x
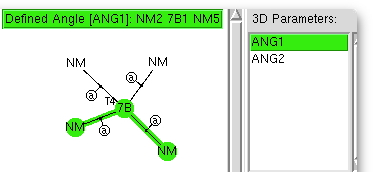
Five things you did not know about (fork) handles.
March 18th, 2014OK, you have to be British to understand the pun in the title, a famous comedy skit about four candles. Back to science, and my mention of some crystal data now having a DOI in the previous post. I thought it might be fun to replicate the contents of one of my ACS slides here.